Physical Activity in Stage III Colon Cancer: CALGB/SWOG 80702 (Alliance)
- PMID: 35944235
- PMCID: PMC9839249
- DOI: 10.1200/JCO.22.00171
Physical Activity in Stage III Colon Cancer: CALGB/SWOG 80702 (Alliance)
Abstract
Purpose: To determine the specific types, durations, and intensities of recreational physical activity associated with the greatest improvements in disease-free survival (DFS) of patients with colon cancer.
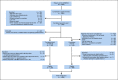
Methods: We conducted a prospective cohort study nested within a randomized multicenter trial of stage III colon cancer that compared 3 versus 6 months of fluorouracil, leucovorin, and oxaliplatin with or without celecoxib. We measured recreational physical activity in the first 3 months of chemotherapy and again 6 months after completion of chemotherapy. The primary end point was DFS.
Results: During a median follow-up of 5.9 years, 457 of 1,696 patients experienced disease recurrence or death. For total recreational physical activity volume, the 3-year DFS was 76.5% with < 3.0 metabolic equivalent task hours per week (MET-h/wk) and 87.1% with ≥ 18.0 MET-h/wk (risk difference [RD], 10.6%; 95% CI, 4.7 to 19.4; P < .001). For light-intensity to moderate-intensity activities, the 3-year DFS was 65.7% with 0.0 h/wk and 87.1% with ≥ 1.5 h/wk (RD, 21.4%; 95% CI, 9.2 to 37.1; P < .001). For vigorous-intensity activity, the 3-year DFS was 76.0% with 0.0 h/wk and 86.0% with ≥ 1.0 h/wk (RD, 10.0%; 95% CI, 4.5 to 18.9; P < .001). For brisk walking, the 3-year DFS was 81.7% with < 1.0 h/wk and 88.4% with ≥ 3.0 h/wk (RD, 6.7%; 95% CI, 3.0 to 13.8; P < .001). For muscle strengthening activity, the 3-year DFS was 81.8% with 0.0 h/wk and 88.8% for ≥ 0.5 h/wk (RD, 7.0%; 95% CI, 3.1 to 14.2; P = .003).
Conclusion: Among patients with stage III colon cancer enrolled in a trial of postoperative treatment, larger volumes of recreational physical activity, longer durations of light- to moderate-intensity aerobic physical activity, or any vigorous-intensity aerobic physical activity were associated with the greatest improvements in DFS.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures

References
-
- Siegel RL, Miller KD, Goding Sauer A, et al. : Colorectal cancer statistics, 2020. CA Cancer J Clin 70:145-164, 2020 - PubMed
-
- Moertel CG, Fleming TR, Macdonald JS, et al. : Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 322:352-358, 1990 - PubMed
-
- Andre T, Boni C, Mounedji-Boudiaf L, et al. : Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350:2343-2351, 2004 - PubMed
-
- Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. : Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Clin Oncol 24:3535-3541, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- UG1 CA233290/CA/NCI NIH HHS/United States
- UG1 CA233320/CA/NCI NIH HHS/United States
- UG1 CA233234/CA/NCI NIH HHS/United States
- UG1 CA189974/CA/NCI NIH HHS/United States
- R00 CA218603/CA/NCI NIH HHS/United States
- U54 GM104940/GM/NIGMS NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- UG1 CA189954/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- UG1 CA233196/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- UG1 CA233253/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- UG1 CA233339/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- UG1 CA233341/CA/NCI NIH HHS/United States
- UG1 CA233337/CA/NCI NIH HHS/United States
- U10 CA180863/CA/NCI NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- UG1 CA233163/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

