Solid-State Microwave Drying for Medical Cannabis Inflorescences: A Rapid and Controlled Alternative to Traditional Drying
- PMID: 35944268
- PMCID: PMC10874826
- DOI: 10.1089/can.2022.0051
Solid-State Microwave Drying for Medical Cannabis Inflorescences: A Rapid and Controlled Alternative to Traditional Drying
Abstract
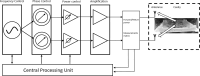
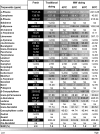
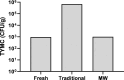
Introduction: As the medical use of Cannabis is evolving there is a greater demand for high-quality products for patients. One of the main steps in the manufacturing process of medical Cannabis is drying. Most current drying methods in the Cannabis industry are relatively slow and inefficient processes. Materials and Methods: This article presents a drying method based on solid-state microwave (MW) that provides fast and uniform drying, and examines its efficiency for drying Cannabis inflorescences compared with the traditional drying method. We assessed 67 cannabinoids and 36 terpenoids in the plant in a range of drying temperatures (40°C, 50°C, 60°C, and 80°C). The identification and quantification of these secondary metabolites were done by chromatography methods. Results: This method resulted in a considerable reduction of drying time, from several days to a few hours. The multiple frequency-phase combination states of the system allowed control and prediction of moisture levels during drying, thus preventing overdrying. A drying temperature of 50°C provided the most effective results in terms of both short drying time and preservation of the composition of the secondary metabolites compared with traditional drying. At 50°C, the chemical profile of phytocannabinoids and terpenoids was best kept to that of the original plant before drying, suggesting less degradation by chemical reactions such as decarboxylation. The fast-drying time also reduced the susceptibility of the plant to microbial contamination. Conclusion: Our results support solid-state MW drying as an effective postharvest step to quickly dry the plant material for improved downstream processing with a minimal negative impact on product quality.
Keywords: drying technology; medical Cannabis; microwave; phytocannabinoids; terpenoids.
Conflict of interest statement
No competing financial interests exist.
Figures

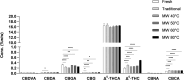

Similar articles
-
Efficient Capture of Cannabis Terpenes in Olive Oil during Microwave-Assisted Cannabinoid Decarboxylation.Molecules. 2024 Feb 18;29(4):899. doi: 10.3390/molecules29040899. Molecules. 2024. PMID: 38398651 Free PMC article.
-
The influence of drying and storage conditions on the volatilome and cannabinoid content of Cannabis sativa L. inflorescences.Anal Bioanal Chem. 2024 Jul;416(16):3797-3809. doi: 10.1007/s00216-024-05321-w. Epub 2024 May 3. Anal Bioanal Chem. 2024. PMID: 38702447 Free PMC article.
-
Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC-MS and LC-HRMS (q-exactive orbitrap®) approach.J Pharm Biomed Anal. 2018 Feb 20;150:208-219. doi: 10.1016/j.jpba.2017.11.073. Epub 2017 Dec 22. J Pharm Biomed Anal. 2018. PMID: 29247961
-
Post-Harvest Operations to Generate High-Quality Medicinal Cannabis Products: A Systemic Review.Molecules. 2022 Mar 6;27(5):1719. doi: 10.3390/molecules27051719. Molecules. 2022. PMID: 35268820 Free PMC article.
-
Vapor Pressure, Vaping, and Corrections to Misconceptions Related to Medical Cannabis' Active Pharmaceutical Ingredients' Physical Properties and Compositions.Cannabis Cannabinoid Res. 2023 Jun;8(3):414-425. doi: 10.1089/can.2021.0173. Epub 2022 Apr 18. Cannabis Cannabinoid Res. 2023. PMID: 35442765 Free PMC article. Review.
Cited by
-
Aquaphotomics study of fresh cannabis inflorescence: near infrared spectral analysis of water matrix structures.Anal Bioanal Chem. 2025 Feb;417(4):747-760. doi: 10.1007/s00216-024-05685-z. Epub 2024 Dec 9. Anal Bioanal Chem. 2025. PMID: 39652218 Free PMC article.
-
In Pursuit of Optimal Quality: Cultivar-Specific Drying Approaches for Medicinal Cannabis.Plants (Basel). 2024 Apr 8;13(7):1049. doi: 10.3390/plants13071049. Plants (Basel). 2024. PMID: 38611577 Free PMC article.
-
Improved Long-Term Preservation of Cannabis Inflorescence by Utilizing Integrated Pre-Harvest Hexanoic Acid Treatment and Optimal Post-Harvest Storage Conditions.Plants (Basel). 2024 Mar 30;13(7):992. doi: 10.3390/plants13070992. Plants (Basel). 2024. PMID: 38611521 Free PMC article.
-
Optimization of Trimming Techniques for Enhancing Cannabinoid and Terpene Content in Medical Cannabis Inflorescences.Med Cannabis Cannabinoids. 2024 Jun 27;7(1):111-118. doi: 10.1159/000539192. eCollection 2024 Jan-Dec. Med Cannabis Cannabinoids. 2024. PMID: 39015609 Free PMC article.
References
-
- ElSohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78:539–548. - PubMed
-
- Maccallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12–19. - PubMed
-
- ElSohly M, Gul W.. Constituents of Cannabis sativa. In: Pertwee R, ed. Handbook of cannabis. Oxford University Press: Oxford, 2014:3–22.
-
- Russo EB, Marcu J. Cannabis pharmacology: the usual suspects and a few promising leads. Adv Pharmacol. 2017;80:67–134. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
