Mechanistic Investigations into Amination of Unactivated Arenes via Cation Radical Accelerated Nucleophilic Aromatic Substitution
- PMID: 35944280
- PMCID: PMC10037305
- DOI: 10.1021/jacs.2c04577
Mechanistic Investigations into Amination of Unactivated Arenes via Cation Radical Accelerated Nucleophilic Aromatic Substitution
Abstract
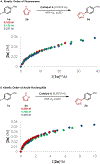
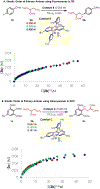
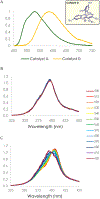
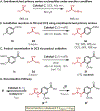
A mechanistic investigation into the amination of electron-neutral and electron-rich arenes using organic photoredox catalysis is presented. Kinetic and computational data support rate-limiting nucleophilic addition into an arene cation radical using both azole and primary amine nucleophiles. This finding is consistent with both fluoride and alkoxide nucleofuges, supporting a unified mechanistic picture using cation radical accelerated nucleophilic aromatic substitution (CRA-SNAr). Electrochemistry and time-resolved fluorescence spectroscopy confirm the key role solvents play in enabling selective arene oxidation in the presence of amines. The synthetic limitations of xanthylium salts are elucidated via photophysical studies. An alternative catalyst scaffold with improved turnover numbers is presented.
Figures




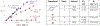

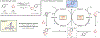


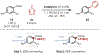


References
-
- Terrier F Modern Nucleophilic Aromatic Substitution; Wiley-VCH: Weinheim, 2013.
-
- Błaziak K; Danikiewicz W; Mąkosza M How Does Nucleophilic Aromatic Substitution Really Proceed in Nitroarenes? Computational Prediction and Experimental Verification. J. Am. Chem. Soc 2016, 138, 7276–7281. - PubMed
-
- Brown DG; Boström J Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone? J. Med. Chem 2016, 59, 4443–4458. - PubMed
-
- Zhang T-T; Jia J-F; Wu H-S Substituent and Solvent Effects on Electronic Structure and Spectral Property of ReCl(CO)3(N∧N) (N∧N = Glyoxime): DFT and TDDFT Theoretical Studies. J. Phys. Chem. A 2010, 114, 12251–12257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

