Harmonization of SARS-CoV-2 reverse transcription quantitative PCR tests to the first WHO international standard for SARS-CoV-2 RNA
- PMID: 35944343
- PMCID: PMC9287539
- DOI: 10.1016/j.jcv.2022.105242
Harmonization of SARS-CoV-2 reverse transcription quantitative PCR tests to the first WHO international standard for SARS-CoV-2 RNA
Abstract
Background: Cycle threshold (Ct) values from SARS-CoV-2 reverse transcription quantitative PCR (RT-qPCR) tests are used to measure viral burden. Calibration to the First WHO International Standard for SARS-CoV-2 RNA may improve quantitative inter-assay agreement.
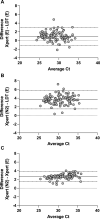
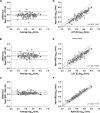
Methods: WHO standard was tested using four emergency use authorized RT-qPCRs to generate calibration curves and evaluate Ct value differences. Harmonization of two assays, Cepheid Xpert Xpress SARS-CoV-2 targeting E and nucleocapsid (N2) [Xpert (E) and Xpert (N2)] and a laboratory-developed test targeting E [LDT (E)], was assessed using 93 positive upper respiratory samples. Platform (target) pairs were compared via Bland-Altman analysis and Passing-Bablok regression.
Results: Ct values with the WHO standard were comparable across platforms and targets, except Xpert (N2) for which the mean difference was a median of 3.68 cycles (Interquartile Range, IQR = 3.23 to 3.76 cycles) greater than other platform (target) pairs. Using clinical samples, the mean difference of Xpert (N2) to LDT (E) was 3.64 cycles (95% Confidence Interval, CI =1.51 to 5.76). After calibration, the mean difference of Xpert (N2) to LDT (E) was 0.08 log10 IU/mL (95% CI = -0.56 to 0.71) and the regression was y = 1.00x * 0.08 (95% CI slope = 0.93 to 1.07, 95% CI intercept = 0.28 to 0.42).
Conclusions: Calibration to the WHO standard resulted in the harmonization of two RT-qPCR tests, whereas analysis by Ct value alone may have led to erroneous quantitation. Harmonization to the WHO standard has the potential to improve the generalizability of clinical associations with SARS-CoV-2 RNA levels.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Clinical Evaluation of BioFire COVID-19 Test, BioFire Respiratory Panel 2.1, and Cepheid Xpert Xpress SARS-CoV-2 Assays for Sample-to-Answer Detection of SARS-CoV-2.Genes (Basel). 2023 Jan 16;14(1):233. doi: 10.3390/genes14010233. Genes (Basel). 2023. PMID: 36672974 Free PMC article.
-
Application of digital PCR to determine the reliability of Xpert Xpress SARS-CoV-2 assay with envelope (E) gene negative and nucleocapsid (N2) gene positive results.Diagn Microbiol Infect Dis. 2022 Aug;103(4):115726. doi: 10.1016/j.diagmicrobio.2022.115726. Epub 2022 May 20. Diagn Microbiol Infect Dis. 2022. PMID: 35691105 Free PMC article.
-
Sample pooling on the Cepheid Xpert® Xpress SARS-CoV-2 assay.Diagn Microbiol Infect Dis. 2021 Feb;99(2):115238. doi: 10.1016/j.diagmicrobio.2020.115238. Epub 2020 Oct 15. Diagn Microbiol Infect Dis. 2021. PMID: 33171384 Free PMC article.
-
Diagnostic accuracy of the Cepheid Xpert Xpress and the Abbott ID NOW assay for rapid detection of SARS-CoV-2: A systematic review and meta-analysis.J Med Virol. 2021 Jul;93(7):4523-4531. doi: 10.1002/jmv.26994. Epub 2021 May 3. J Med Virol. 2021. PMID: 33913533 Free PMC article.
-
Cycle Threshold Values from Severe Acute Respiratory Syndrome Coronavirus-2 Reverse Transcription-Polymerase Chain Reaction Assays: Interpretation and Potential Use Cases.Clin Lab Med. 2022 Jun;42(2):237-248. doi: 10.1016/j.cll.2022.02.003. Epub 2022 Feb 21. Clin Lab Med. 2022. PMID: 35636824 Free PMC article. Review.
Cited by
-
Standardization of SARS-CoV-2 Nucleic Acid Amplification Techniques by Calibration and Quantification to the First WHO International Standard for SARS-CoV-2 RNA.Int J Microbiol. 2023 Feb 17;2023:7803864. doi: 10.1155/2023/7803864. eCollection 2023. Int J Microbiol. 2023. PMID: 36846152 Free PMC article.
-
Standardization and Comparison of Emergency Use Authorized COVID-19 Assays and Testing Laboratories.medRxiv [Preprint]. 2023 Nov 8:2023.11.08.23297633. doi: 10.1101/2023.11.08.23297633. medRxiv. 2023. PMID: 37986832 Free PMC article. Preprint.
-
Testing for SARS-CoV-2: lessons learned and current use cases.Clin Microbiol Rev. 2024 Jun 13;37(2):e0007223. doi: 10.1128/cmr.00072-23. Epub 2024 Mar 15. Clin Microbiol Rev. 2024. PMID: 38488364 Free PMC article. Review.
-
Evaluation of Acebilustat, a Selective Inhibitor of Leukotriene B4 Biosynthesis, for Treatment of Outpatients With Mild-Moderate Coronavirus Disease 2019: A Randomized, Double-Blind, Placebo-Controlled Phase 2 Trial.Clin Infect Dis. 2023 Jul 26;77(2):186-193. doi: 10.1093/cid/ciad187. Clin Infect Dis. 2023. PMID: 36996150 Free PMC article. Clinical Trial.
References
-
- Rhoads D.D., Pinsky B.A. The truth about SARS-CoV-2 cycle threshold values is rarely pure and never simple. Clin. Chem. 2021;68:16–18. - PubMed
-
- Rhoads D., Peaper D.R., She R.C., Nolte F.S., Wojewoda C.M., Anderson N.W., Pritt B.S. College of American pathologists (CAP) microbiology committee perspective: caution must be used in interpreting the cycle threshold (Ct) value. Clin. Infect. Dis. 2021;72:e685–e686. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

