Enhanced exon skipping and prolonged dystrophin restoration achieved by TfR1-targeted delivery of antisense oligonucleotide using FORCE conjugation in mdx mice
- PMID: 35944903
- PMCID: PMC9723632
- DOI: 10.1093/nar/gkac641
Enhanced exon skipping and prolonged dystrophin restoration achieved by TfR1-targeted delivery of antisense oligonucleotide using FORCE conjugation in mdx mice
Abstract
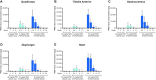
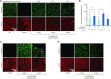
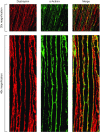
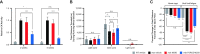
Current therapies for Duchenne muscular dystrophy (DMD) use phosphorodiamidate morpholino oligomers (PMO) to induce exon skipping in the dystrophin pre-mRNA, enabling the translation of a shortened but functional dystrophin protein. This strategy has been hampered by insufficient delivery of PMO to cardiac and skeletal muscle. To overcome these limitations, we developed the FORCETM platform consisting of an antigen-binding fragment, which binds the transferrin receptor 1, conjugated to an oligonucleotide. We demonstrate that a single dose of the mouse-specific FORCE-M23D conjugate enhances muscle delivery of exon skipping PMO (M23D) in mdx mice, achieving dose-dependent and robust exon skipping and durable dystrophin restoration. FORCE-M23D-induced dystrophin expression reached peaks of 51%, 72%, 62%, 90% and 77%, of wild-type levels in quadriceps, tibialis anterior, gastrocnemius, diaphragm, and heart, respectively, with a single 30 mg/kg PMO-equivalent dose. The shortened dystrophin localized to the sarcolemma, indicating expression of a functional protein. Conversely, a single 30 mg/kg dose of unconjugated M23D displayed poor muscle delivery resulting in marginal levels of exon skipping and dystrophin expression. Importantly, FORCE-M23D treatment resulted in improved functional outcomes compared with administration of unconjugated M23D. Our results suggest that FORCE conjugates are a potentially effective approach for the treatment of DMD.
Plain language summary
The biggest problem confronting oligonucleotide therapeutics is a lack of compounds capable of targeting compounds to diseased tissues. This paper reports a major advance targeting the transferrin receptor to increase the delivery of morpholine oligomers to muscle cells in vivo. This work suggests the possibility for improved treatments of muscular dystrophy and other diseases.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




Similar articles
-
AOC 1044 induces exon 44 skipping and restores dystrophin protein in preclinical models of Duchenne muscular dystrophy.Nucleic Acids Res. 2025 Mar 20;53(6):gkaf241. doi: 10.1093/nar/gkaf241. Nucleic Acids Res. 2025. PMID: 40183632 Free PMC article.
-
Bubble liposomes and ultrasound exposure improve localized morpholino oligomer delivery into the skeletal muscles of dystrophic mdx mice.Mol Pharm. 2014 Mar 3;11(3):1053-61. doi: 10.1021/mp4004755. Epub 2014 Jan 27. Mol Pharm. 2014. PMID: 24433046
-
Nonclinical Exon Skipping Studies with 2'-O-Methyl Phosphorothioate Antisense Oligonucleotides in mdx and mdx-utrn-/- Mice Inspired by Clinical Trial Results.Nucleic Acid Ther. 2019 Apr;29(2):92-103. doi: 10.1089/nat.2018.0759. Epub 2019 Jan 23. Nucleic Acid Ther. 2019. PMID: 30672725 Free PMC article.
-
Molecular correction of Duchenne muscular dystrophy by splice modulation and gene editing.RNA Biol. 2021 Jul;18(7):1048-1062. doi: 10.1080/15476286.2021.1874161. Epub 2021 Jan 20. RNA Biol. 2021. PMID: 33472516 Free PMC article. Review.
-
Skipping multiple exons of dystrophin transcripts using cocktail antisense oligonucleotides.Nucleic Acid Ther. 2014 Feb;24(1):57-68. doi: 10.1089/nat.2013.0451. Epub 2013 Dec 31. Nucleic Acid Ther. 2014. PMID: 24380394 Review.
Cited by
-
A Review of Protein- and Peptide-Based Chemical Conjugates: Past, Present, and Future.Pharmaceutics. 2023 Feb 10;15(2):600. doi: 10.3390/pharmaceutics15020600. Pharmaceutics. 2023. PMID: 36839922 Free PMC article. Review.
-
Evolution of antisense oligonucleotides: navigating nucleic acid chemistry and delivery challenges.Expert Opin Drug Discov. 2025 Jan;20(1):63-80. doi: 10.1080/17460441.2024.2440095. Epub 2024 Dec 17. Expert Opin Drug Discov. 2025. PMID: 39653607 Review.
-
Conjugation to a transferrin receptor 1-binding Bicycle peptide enhances ASO and siRNA potency in skeletal and cardiac muscles.Nucleic Acids Res. 2025 Apr 10;53(7):gkaf270. doi: 10.1093/nar/gkaf270. Nucleic Acids Res. 2025. PMID: 40207629 Free PMC article.
-
Recent Progress and Challenges in the Development of Antisense Therapies for Myotonic Dystrophy Type 1.Int J Mol Sci. 2022 Nov 1;23(21):13359. doi: 10.3390/ijms232113359. Int J Mol Sci. 2022. PMID: 36362145 Free PMC article. Review.
-
Structure-activity relationship study of mesyl and busyl phosphoramidate antisense oligonucleotides for unaided and PSMA-mediated uptake into prostate cancer cells.Front Chem. 2024 Mar 4;12:1342178. doi: 10.3389/fchem.2024.1342178. eCollection 2024. Front Chem. 2024. PMID: 38501046 Free PMC article.
References
-
- Emery A.E. Population frequencies of inherited neuromuscular diseases–a world survey. Neuromuscul. Disord. 1991; 1:19–29. - PubMed
-
- Gatheridge M.A., Kwon J.M., Mendell J.M., Scheuerbrandt G., Moat S.J., Eyskens F., Rockman-Greenberg C., Drousiotou A., Griggs R.C.. Identifying non-duchenne muscular dystrophy-positive and false negative results in prior duchenne muscular dystrophy newborn screening programs: a review. JAMA Neurol. 2016; 73:111–116. - PubMed
-
- Flanigan K.M., Dunn D.M., von Niederhausern A., Soltanzadeh P., Gappmaier E., Howard M.T., Sampson J.B., Mendell J.R., Wall C., King W.M.et al. .. Mutational spectrum of DMD mutations in dystrophinopathy patients: application of modern diagnostic techniques to a large cohort. Hum. Mutat. 2009; 30:1657–1666. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

