Temporal trends in COVID-19 outcomes among patients with systemic autoimmune rheumatic diseases: from the first wave through the initial Omicron wave
- PMID: 35944947
- PMCID: PMC9939910
- DOI: 10.1136/ard-2022-222954
Temporal trends in COVID-19 outcomes among patients with systemic autoimmune rheumatic diseases: from the first wave through the initial Omicron wave
Abstract
Objectives: To investigate temporal trends in incidence and severity of COVID-19 among patients with systemic autoimmune rheumatic diseases (SARDs) from the first wave through the initial Omicron wave.
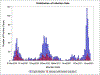
Methods: We conducted a retrospective cohort study investigating COVID-19 outcomes among patientswith SARD systematically identified to have confirmed COVID-19 from 1 March 2020 to 31 January 2022 at Mass General Brigham. We tabulated COVID-19 counts of total and severe cases (hospitalisations or deaths) and compared the proportion with severe COVID-19 by calendar period and by vaccination status. We used logistic regression to estimate the ORs for severe COVID-19 for each period compared with the early COVID-19 period (reference group).
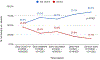
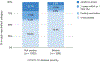
Results: We identified 1449 patients with SARD with COVID-19 (mean age 58.4 years, 75.2% female, 33.9% rheumatoid arthritis). There were 399 (28%) cases of severe COVID-19. The proportion of severe COVID-19 outcomes declined over calendar time (p for trend <0.001); 46% of cases were severe in the early COVID-19 period (1 March 2020-30 June 2020) vs 15% in the initial Omicron wave (17 December 2021-31 January 2022; adjusted OR 0.29, 95% CI 0.19 to 0.43). A higher proportion of those unvaccinated were severe compared with not severe cases (78% vs 60%).
Conclusions: The proportion of patients with SARD with severe COVID-19 has diminished since early in the pandemic, particularly during the most recent time periods, including the initial Omicron wave. Advances in prevention, diagnosis and treatment of COVID-19 may have improved outcomes among patients with SARD.
Keywords: Autoimmune Diseases; Covid-19; Vaccination.
© Author(s) (or their employer(s)) 2022. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: NJP reports consulting fees from FVC Health unrelated to this work. MEW reports research support from Bristol Meyers Squibb, Sanofi, Eli Lilly, Amgen, and consulting fees from Abbvie, Aclaris, Amgen Bristol Myers Squibb, Corevitas, EQRx, Genosco, GlaxoSmithKline, Gilead, Horizon, Johnson & Johnson, Eli Lilly, Pfizer, Roche, Sanofi, Scipher, Set Point, and Tremeau, and stock options in Can-Fite, Inmediz, and Scipher unrelated to this work. ZSW reports research support from Bristol-Myers Squibb and Principia/Sanofi and consulting fees from Viela Bio, Zenas BioPharma, and MedPace. JAS reports research support from Bristol Myers Squibb and consultancy fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum and Pfizer unrelated to this work. All other authors report no competing interests.
Figures
Update of
-
Temporal trends in COVID-19 outcomes among patients with systemic autoimmune rheumatic diseases: From the first wave to Omicron.medRxiv [Preprint]. 2022 Jun 20:2022.06.19.22276599. doi: 10.1101/2022.06.19.22276599. medRxiv. 2022. Update in: Ann Rheum Dis. 2022 Dec;81(12):1742-1749. doi: 10.1136/ard-2022-222954. PMID: 35765565 Free PMC article. Updated. Preprint.
References
-
- Hui DSC, Zumla A. Advances in the epidemiology, clinical features, diagnosis, clinical management and prevention of coronavirus disease 2019. Curr Opin Pulm Med 2022;28:166–73. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

