Should COVID-19 vaccines be mandated in schools? - an international caregiver perspective
- PMID: 35945047
- PMCID: PMC9339978
- DOI: 10.1016/j.vaccine.2022.07.038
Should COVID-19 vaccines be mandated in schools? - an international caregiver perspective
Abstract
Objectives: Caregiver attitudes toward mandating COVID-19 vaccines for their children are poorly understood. We aimed to determine caregiver acceptability of COVID-19 vaccine mandates for schools/daycares and assess if opposition to mandates would result in removal of children from the educational system.
Study design: Perform a cross-sectional, anonymous survey of adult caregivers with children ≤ 18 years presenting to 21 pediatric emergency departments in the United States, Canada, Israel, and Switzerland, November 1st through December 31st, 2021. The primary outcome was caregiver acceptance rates for school vaccine mandates, and the secondary outcomes included factors associated with mandate acceptance and caregiver intention to remove the child from school.
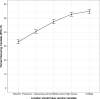
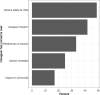
Results: Of 4,393 completed surveys, 37% of caregivers were opposed to any school vaccine mandate. Caregiver acceptance was lowest for daycare settings (33%) and increased as the child's level of education increased, college (55%). 26% of caregivers report a high likelihood (score of 8-10 on 0-10 scale) to remove their child from school if the vaccine became mandatory. Child safety was caregivers' greatest concern over vaccine mandates. A multivariable model demonstrated intent to vaccinate their child for COVID-19 (OR = 8.9, 95% CI 7.3 to 10.8; P < 0.001) and prior COVID-19 vaccination for the caregiver (OR = 3.8, 95% CI 3.0 to 4.9; P < 0.001) had the greatest odds of increasing mandate acceptance for any school level.
Conclusions: Many caregivers are resistant to COVID-19 vaccine mandates for schools, and acceptance varies with school level. One-fourth of caregivers plan to remove their child from the educational system if vaccines become mandated.
Copyright © 2022. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Woodworth K, Moulia D, Collins JP, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine in Children Aged 5–11 Years — United States, November 2021. Centers for Disease Control and Prevention. Updated November 10, 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7045e1.htm#suggestedcitation.[Accessed Februrary 17, 2022]. - PubMed
-
- Wallace M, Woodworth, K, and Gargano J, et al. The Advisory Committee Immunization Practices' Intermin Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine in Adolescents Aged 12-15 Years. Centers for Disease Control and Prevention Updated 5/14/21. https://www.cdc.gov/mmwr/volumes/70/wr/mm7020e1.htm [Accessed September 23, 2021].
-
- Summary of National Advisory Committee on Immunization (NACI) Statement of January 25, 2022: Updated Recommendations on the Use of COVID-19 Vaccines in Children 5 to 11 years of age. Public Health Agency of Canada. 2022. https://www.canada.ca/content/dam/phac-aspc/documents/services/immunizat.... [Accessed 29 April 2022].
-
- Shamir J. New York Times; 2021. (Israeli experts approve vaccinations for children ages 5 to 11). https://www.nytimes.com/2021/11/10/world/middleeast/israeli-covid-vaccin....
-
- Swissmedic approves COVID-19 vaccine from Pfizer/BioNTech for children aged 5 to 11 years. Swissmedic 2021. https://www.swissmedic.ch/swissmedic/en/home/news/coronavirus-covid-19/c.... [Accessed 29 April 2022 ].

