Protection of Omicron sub-lineage infection against reinfection with another Omicron sub-lineage
- PMID: 35945213
- PMCID: PMC9362989
- DOI: 10.1038/s41467-022-32363-4
Protection of Omicron sub-lineage infection against reinfection with another Omicron sub-lineage
Abstract
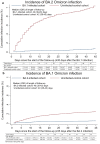
There is significant genetic distance between SARS-CoV-2 Omicron (B.1.1.529) variant BA.1 and BA.2 sub-lineages. This study investigates immune protection of infection with one sub-lineage against reinfection with the other sub-lineage in Qatar during a large BA.1 and BA.2 Omicron wave, from December 19, 2021 to March 21, 2022. Two national matched, retrospective cohort studies are conducted to estimate effectiveness of BA.1 infection against reinfection with BA.2 (N = 20,994; BA.1-against-BA.2 study), and effectiveness of BA.2 infection against reinfection with BA.1 (N = 110,315; BA.2-against-BA.1 study). Associations are estimated using Cox proportional-hazards regression models after multiple imputation to assign a sub-lineage status for cases with no sub-lineage status (using probabilities based on the test date). Effectiveness of BA.1 infection against reinfection with BA.2 is estimated at 94.2% (95% CI: 89.2-96.9%). Effectiveness of BA.2 infection against reinfection with BA.1 is estimated at 80.9% (95% CI: 73.1-86.4%). Infection with the BA.1 sub-lineage appears to induce strong, but not full immune protection against reinfection with the BA.2 sub-lineage, and vice versa, for at least several weeks after the initial infection.
© 2022. The Author(s).
Conflict of interest statement
Dr. Butt has received institutional grant funding from Gilead Sciences unrelated to the work presented in this paper. Otherwise, we declare no competing interests.
Figures

References
-
- World Health Organization. Tracking SARS-CoV-2 variants. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (2021).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

