Pro-oxidant response and accelerated ferroptosis caused by synergetic Au(I) release in hypercarbon-centered gold(I) cluster prodrugs
- PMID: 35945240
- PMCID: PMC9363434
- DOI: 10.1038/s41467-022-32474-y
Pro-oxidant response and accelerated ferroptosis caused by synergetic Au(I) release in hypercarbon-centered gold(I) cluster prodrugs
Abstract
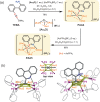
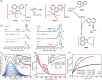
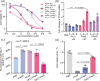
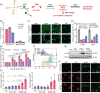
Medicinal applications of gold complexes have recently attracted attention due to their innovative antitumor mechanisms. In this work, two hypercoordinated carbon-centered gold clusters PAA4 and PAA5 are quantitatively synthesized by an intramolecular 6-exo-dig cyclization of polymetalated precursors. The on-bench and in vitro experimental studies demonstrate that the characteristic hypercarbon-tetragold(I) multi-center bonding in PAA4 and PAA5 not only guarantees their stability under common physiological conditions, but also facilitates a glutathione (GSH)-triggered prompt and synergetic release of active Au(I) ions in the GSH-overexpressed and acidic microenvironment of human bladder cancer EJ cells. The instantly massive release of coordination unsaturated Au(I) ions causes the efficient inhibition of thioredoxin reductases and then induces a rapid pro-oxidant response, consequently causing the occurrence of accelerated ferroptosis of EJ cells. As a result, these hypercarbon-centered gold(I) cluster prodrugs show high cytotoxicity to bladder cancer cell lines and thus exhibit a significant inhibition effect towards bladder tumors in vivo. Correlation of the synergetic domino dissociation of carbon-polymetal multi-center bonding in metal clusters with the accelerated ferroptosis of cancer cells provides a strategy for metallo-prodrugs and opens a broader prospect for the biological application of metal cluster compounds.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Synergistic Enhancement of Ferroptosis via Mitochondrial Accumulation and Photodynamic-Controlled Release of an Organogold(I) Cluster Prodrug.J Am Chem Soc. 2025 Feb 5;147(5):4482-4492. doi: 10.1021/jacs.4c15820. Epub 2025 Jan 24. J Am Chem Soc. 2025. PMID: 39851086
-
Delivery of a system xc- inhibitor by a redox-responsive levodopa prodrug nanoassembly for combination ferrotherapy.J Mater Chem B. 2021 Sep 15;9(35):7172-7181. doi: 10.1039/d1tb00742d. J Mater Chem B. 2021. PMID: 34369535
-
Reactive oxygen species / photothermal therapy dual-triggered biomimetic gold nanocages nanoplatform for combination cancer therapy via ferroptosis and tumor-associated macrophage repolarization mechanism.J Colloid Interface Sci. 2022 Jan 15;606(Pt 2):1950-1965. doi: 10.1016/j.jcis.2021.09.160. Epub 2021 Sep 29. J Colloid Interface Sci. 2022. PMID: 34695762
-
Application of natural products for inducing ferroptosis in tumor cells.Biotechnol Appl Biochem. 2022 Feb;69(1):190-197. doi: 10.1002/bab.2096. Epub 2021 Jan 21. Biotechnol Appl Biochem. 2022. PMID: 33393679 Review.
-
Directly targeting glutathione peroxidase 4 may be more effective than disrupting glutathione on ferroptosis-based cancer therapy.Biochim Biophys Acta Gen Subj. 2020 Apr;1864(4):129539. doi: 10.1016/j.bbagen.2020.129539. Epub 2020 Jan 18. Biochim Biophys Acta Gen Subj. 2020. PMID: 31958545 Review.
Cited by
-
Ferroptosis resistance in cancer cells: nanoparticles for combination therapy as a solution.Front Pharmacol. 2024 Jun 19;15:1416382. doi: 10.3389/fphar.2024.1416382. eCollection 2024. Front Pharmacol. 2024. PMID: 38962305 Free PMC article. Review.
-
Plant-derived nanovesicles as an emerging platform for cancer therapy.Acta Pharm Sin B. 2024 Jan;14(1):133-154. doi: 10.1016/j.apsb.2023.08.033. Epub 2023 Sep 3. Acta Pharm Sin B. 2024. PMID: 38239235 Free PMC article. Review.
-
Gold(I) N-Heterocyclic Carbene Complexes as Ferroptosis Inducing Anticancer Agents.ACS Med Chem Lett. 2025 Apr 24;16(5):856-864. doi: 10.1021/acsmedchemlett.5c00096. eCollection 2025 May 8. ACS Med Chem Lett. 2025. PMID: 40365416
-
The core genes of cuproptosis assists in discerning prognostic and immunological traits of clear cell renal cell carcinoma.Front Oncol. 2022 Sep 21;12:925411. doi: 10.3389/fonc.2022.925411. eCollection 2022. Front Oncol. 2022. PMID: 36212447 Free PMC article.
-
Synergistic ferroptosis-starvation therapy for bladder cancer based on hyaluronic acid modified metal-organic frameworks.Bioeng Transl Med. 2023 Mar 22;8(3):e10515. doi: 10.1002/btm2.10515. eCollection 2023 May. Bioeng Transl Med. 2023. PMID: 37206228 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

