SOX2 expression in the pathogenesis of premalignant lesions of the uterine cervix: its histo-topographical distribution distinguishes between low- and high-grade CIN
- PMID: 35945296
- PMCID: PMC9726813
- DOI: 10.1007/s00418-022-02145-6
SOX2 expression in the pathogenesis of premalignant lesions of the uterine cervix: its histo-topographical distribution distinguishes between low- and high-grade CIN
Abstract
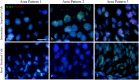
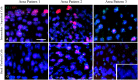
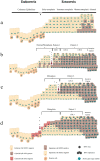
SOX2 expression in high-grade cervical intraepithelial neoplasia (CIN3) and cervical squamous cell carcinoma is increased compared to that in the normal cervical epithelium. However, data on the expression and histological distribution of SOX2 in squamous epithelium during progression of CIN are largely lacking. We studied SOX2 expression throughout the epithelium in 53 cases of CIN1, 2, and 3. In general, SOX2 expression increased and expanded from basal/parabasal to the intermediate/superficial compartment during early stages of progression of CIN. An unexpected, specific expression pattern was found in areas classified as CIN2 and CIN3. This pattern was characterized by the absence or low expression of SOX2 in the basal/parabasal compartment and variable levels in the intermediate and superficial compartments. It was significantly associated with CIN3 (p = 0.009), not found in CIN1 and only seen in part of the CIN2 lesions. When the different patterns were correlated with the genetic make-up and presence of HPV, the CIN3-related pattern contained HPV-positive cells in the basal/parabasal cell compartment that were disomic. This is in contrast to the areas exhibiting the CIN1 and CIN2 related patterns, which frequently exhibited aneusomic cells. Based on their SOX2 localisation pattern, CIN1 and CIN2 could be delineated from CIN3. These data shed new light on the pathogenesis and dynamics of progression in premalignant cervical lesions, as well as on the target cells in the epithelium for HPV infection.
Keywords: CIN; Cervical preneoplasia; Genetic aberrations; HPV infection; SOX2 distribution; Squamous intraepithelial lesions.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict interests.
Figures




References
-
- Chang X, Zhang J, Huang C, Pang X, Luo Q, Zhang H, Zhang S. Sex-determining region Y-related high mobility group box (SOX)-2 is overexpressed in cervical squamous cell carcinoma and contributes cervical cancer cell migration and invasion in vitro. Tumour Biol. 2015;36(10):7725–7733. doi: 10.1007/s13277-015-3450-y. - DOI - PubMed
-
- Chen Y, Shi L, Zhang L, Li R, Liang J, Yu W, Sun L, Yang X, Wang Y, Zhang Y, Shang Y (2008) The molecular mechanism governing the oncogenic potential of SOX2 in breast cancer. J Biol Chem 283 (26):17969–17978. 10.1074/jbc.M802917200 - PubMed
-
- Chen EY, Tran A, Raho CJ, Birch CM, Crum CP, Hirsch MS (2010) Histological 'progression' from low (LSIL) to high (HSIL) squamous intraepithelial lesion is an uncommon event and an indication for quality assurance review. Mod Pathol 23 (8):1045–1051. 10.1038/modpathol.2010.85 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

