The Pharmacological Effects of Phenylephrine are Indirect, Mediated by Noradrenaline Release from the Cytoplasm
- PMID: 35945308
- PMCID: PMC9546997
- DOI: 10.1007/s11064-022-03681-2
The Pharmacological Effects of Phenylephrine are Indirect, Mediated by Noradrenaline Release from the Cytoplasm
Abstract
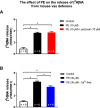
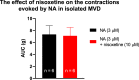
Phenylephrine (PE) is a canonical α1-adrenoceptor-selective agonist. However, unexpected effects of PE have been observed in preclinical and clinical studies, that cannot be easily explained by its actions on α1-adrenoceptors. The probability of the involvement of α2- and β-adrenoceptors in the effect of PE has been raised. In addition, our earlier study observed that PE released noradrenaline (NA) in a [Ca2+]o-independent manner. To elucidate this issue, we have investigated the effects of PE on [3H]NA release and α1-mediated smooth muscle contractions in the mouse vas deferens (MVD) as ex vivo preparation. The release experiments were designed to assess the effects of PE at the presynaptic terminal, whereas smooth muscle isometric contractions in response to electrical field stimulation were used to measure PE effect postsynaptically. Our results show that PE at concentrations between 0.3 and 30 µM significantly enhanced the resting release of [3H]NA in a [Ca2+]o-independent manner. In addition, prazosin did not affect the release of NA evoked by PE. On the contrary, PE-evoked smooth muscle contractions were inhibited by prazosin administration indicating the α1-adrenoceptor-mediated effect. When the function of the NA transporter (NAT) was attenuated with nisoxetine, PE failed to release NA and the contractions were reduced by approximately 88%. The remaining part proved to be prazosin-sensitive. The present work supports the substantial indirect effect of PE which relays on the cytoplasmic release of NA, which might explain the reported side effects for PE.
Keywords: Cytoplasmic origin; Indirect action; Noradrenaline release; Noradrenaline transporter; Phenylephrine; α1-Adrenoceptor.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Ahlquist RP. A study of the adrenotropic receptors. Am J Physiol. 1948;153(3):586–600. - PubMed
-
- Minneman KP, et al. Selectivity of agonists for cloned alpha 1-adrenergic receptor subtypes. Mol Pharmacol. 1994;46(5):929–936. - PubMed
-
- Hieble JP, et al. International Union of Pharmacology. X. Recommendation for nomenclature of alpha 1-adrenoceptors: consensus update. Pharmacol Rev. 1995;47(2):267–470. - PubMed
-
- Vizi ES. Compounds acting on alpha 1- and alpha 2- adrenoceptors: agonists and antagonists. Med Res Rev. 1986;6(4):431–449. - PubMed
-
- Goodman & Gilman’s . The pharmacological basis of therapeutics. 13. New York: McGraw Hill; 2017.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

