14-Deoxygarcinol improves insulin sensitivity in high-fat diet-induced obese mice via mitigating NF-κB/Sirtuin 2-NLRP3-mediated adipose tissue remodeling
- PMID: 35945312
- PMCID: PMC9889782
- DOI: 10.1038/s41401-022-00958-8
14-Deoxygarcinol improves insulin sensitivity in high-fat diet-induced obese mice via mitigating NF-κB/Sirtuin 2-NLRP3-mediated adipose tissue remodeling
Abstract
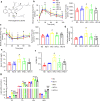
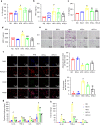
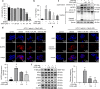
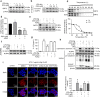
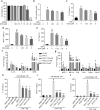
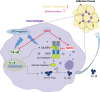
Interleukin (IL)-1β is a culprit of adipose tissue inflammation, which in turn causes systematic inflammation and insulin resistance in obese individuals. IL-1β is mainly produced in monocytes and macrophages and marginally in adipocytes, through cleavage of the inactive pro-IL-1β precursor by caspase-1, which is activated via the NLRP3 inflammasome complex. The nuclear factor-κB (NF-κB) transcription factor is the master regulator of inflammatory responses. Brindle berry (Garcinia cambogia) has been widely used as health products for treating obesity and related metabolic disorders, but its active principles remain unclear. We previously found a series of polyisoprenylated benzophenones from brindle berry with anti-inflammatory activities. In this study we investigated whether 14-deoxygarcinol (DOG), a major polyisoprenylated benzophenone from brindle berry, alleviated adipose tissue inflammation and insulin sensitivity in high-fat diet fed mice. The mice were administered DOG (2.5, 5 mg · kg-1 · d-1, i.p.) for 4 weeks. We showed that DOG injection dose-dependently improved insulin resistance and hyperlipidemia, but not adiposity in high-fat diet-fed mice. We found that DOG injection significantly alleviated adipose tissue inflammation via preventing macrophage infiltration and pro-inflammatory polarization of macrophages, and adipose tissue fibrosis via reducing the abnormal deposition of extracellular matrix. In LPS plus nigericin-stimulated THP-1 macrophages, DOG (1.25, 2.5, 5 μM) dose-dependently suppressed the activation of NLRP3 inflammasome and NF-κB signaling pathway. We demonstrated that DOG bound to and activated the deacetylase Sirtuin 2, which in turn deacetylated and inactivated NLRP3 inflammasome to reduce IL-1β secretion. Moreover, DOG (1.25, 2.5, 5 μM) dose-dependently mitigated inflammatory responses in macrophage conditioned media-treated adipocytes and suppressed macrophage migration toward adipocytes. Taken together, DOG might be a drug candidate to treat metabolic disorders through modulation of adipose tissue remodeling.
Keywords: 14-deoxygarcinol; NLRP3 inflammasome; Sirtuin 2; adipose tissue inflammation; insulin resistance; interleukin-1β.
© 2022. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Ainsliadimer C, a disesquiterpenoid isolated from Ainsliaea macrocephala, ameliorates inflammatory responses in adipose tissue via Sirtuin 1-NLRP3 inflammasome axis.Acta Pharmacol Sin. 2022 Jul;43(7):1780-1792. doi: 10.1038/s41401-021-00797-z. Epub 2021 Nov 17. Acta Pharmacol Sin. 2022. PMID: 34789920 Free PMC article.
-
Chikusetsu saponin IVa ameliorates high fat diet-induced inflammation in adipose tissue of mice through inhibition of NLRP3 inflammasome activation and NF-κB signaling.Oncotarget. 2017 May 9;8(19):31023-31040. doi: 10.18632/oncotarget.16052. Oncotarget. 2017. PMID: 28415686 Free PMC article.
-
A casein hydrolysate protects mice against high fat diet induced hyperglycemia by attenuating NLRP3 inflammasome-mediated inflammation and improving insulin signaling.Mol Nutr Food Res. 2016 Nov;60(11):2421-2432. doi: 10.1002/mnfr.201501054. Epub 2016 Sep 8. Mol Nutr Food Res. 2016. PMID: 27390025
-
Reappraisal of Adipose Tissue Inflammation in Obesity.Adv Exp Med Biol. 2024;1460:297-327. doi: 10.1007/978-3-031-63657-8_10. Adv Exp Med Biol. 2024. PMID: 39287856 Review.
-
NLRP3 Inflammasome Activation in Adipose Tissues and Its Implications on Metabolic Diseases.Int J Mol Sci. 2020 Jun 11;21(11):4184. doi: 10.3390/ijms21114184. Int J Mol Sci. 2020. PMID: 32545355 Free PMC article. Review.
Cited by
-
CLICs Inhibitor IAA94 Alleviates Inflammation and Injury in Septic Liver by Preventing Pyroptosis in Macrophages.Inflammation. 2025 Apr 21. doi: 10.1007/s10753-025-02304-6. Online ahead of print. Inflammation. 2025. PMID: 40259192
-
Identifying potential drugs for treating Cardiovascular-kidney metabolic syndrome via reverse network pharmacology.Front Pharmacol. 2025 Jun 30;16:1627236. doi: 10.3389/fphar.2025.1627236. eCollection 2025. Front Pharmacol. 2025. PMID: 40661082 Free PMC article.
-
Exosomal miRNA-155-5p from M1-polarized macrophages suppresses angiogenesis by targeting GDF6 to interrupt diabetic wound healing.Mol Ther Nucleic Acids. 2023 Nov 10;34:102074. doi: 10.1016/j.omtn.2023.102074. eCollection 2023 Dec 12. Mol Ther Nucleic Acids. 2023. PMID: 38074896 Free PMC article.
-
Adipocyte-expressed SIRT3 manipulates carnitine pool to orchestrate metabolic reprogramming and polarization of macrophages.Cell Death Dis. 2025 May 15;16(1):381. doi: 10.1038/s41419-025-07699-6. Cell Death Dis. 2025. PMID: 40368890 Free PMC article.
-
Frequency of the rs2015 (T>G) and rs2241703 (G>A) polymorphisms in the miRNA-SIRT2 gene in type 2 diabetes mellitus in Saudi Arabia.Saudi Med J. 2023 Apr;44(4):363-367. doi: 10.15537/smj.2023.44.4.20220863. Saudi Med J. 2023. PMID: 37062555 Free PMC article.
References
-
- World Health Organization. Obesity and overweight. 2021. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

