Thoracic perivascular adipose tissue inhibits VSMC apoptosis and aortic aneurysm formation in mice via the secretome of browning adipocytes
- PMID: 35945313
- PMCID: PMC9889802
- DOI: 10.1038/s41401-022-00959-7
Thoracic perivascular adipose tissue inhibits VSMC apoptosis and aortic aneurysm formation in mice via the secretome of browning adipocytes
Abstract
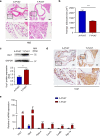
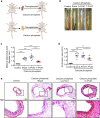
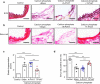
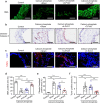
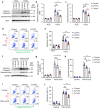
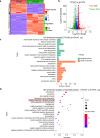
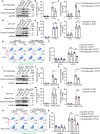
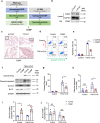
Abdominal aortic aneurysm (AAA) is a dangerous vascular disease without any effective drug therapies so far. Emerging evidence suggests the phenotypic differences in perivascular adipose tissue (PVAT) between regions of the aorta are implicated in the development of atherosclerosis evidenced by the abdominal aorta more vulnerable to atherosclerosis than the thoracic aorta in large animals and humans. The prevalence of thoracic aortic aneurysms (TAA) is much less than that of abdominal aortic aneurysms (AAA). In this study we investigated the effect of thoracic PVAT (T-PVAT) transplantation on aortic aneurysm formation and the impact of T-PVAT on vascular smooth muscle cells. Calcium phosphate-induced mouse AAA model was established. T-PVAT (20 mg) was implanted around the abdominal aorta of recipient mice after removal of endogenous abdominal PVAT (A-PVAT) and calcium phosphate treatment. Mice were sacrificed two weeks after the surgery and the maximum external diameter of infrarenal aorta was measured. We found that T-PVAT displayed a more BAT-like phenotype than A-PVAT; transplantation of T-PVAT significantly attenuated calcium phosphate-induced abdominal aortic dilation and elastic degradation as compared to sham control or A-PVAT transplantation. In addition, T-PVAT transplantation largely preserved smooth muscle cell content in the abdominal aortic wall. Co-culture of T-PVAT with vascular smooth muscle cells (VSMCs) significantly inhibited H2O2- or TNFα plus cycloheximide-induced VSMC apoptosis. RNA sequencing analysis showed that T-PVAT was enriched by browning adipocytes and anti-apoptotic secretory proteins. We further verified that the secretome of mature adipocytes isolated from T-PVAT significantly inhibited H2O2- or TNFα plus cycloheximide-induced VSMC apoptosis. Using proteomic and bioinformatic analyses we identified cartilage oligomeric matrix protein (COMP) as a secreted protein significantly increased in T-PVAT. Recombinant COMP protein significantly inhibited VSMC apoptosis. We conclude that T-PVAT exerts anti-apoptosis effect on VSMCs and attenuates AAA formation, which is possibly attributed to the secretome of browning adipocytes.
Keywords: abdominal aortic aneurysm; apoptosis; browning; perivascular adipose tissue; secretome; vascular smooth muscle cells.
© 2022. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
SM22α-Lineage Perivascular Stromal Cells Contribute to Abdominal Aortic Aneurysm.Circ Res. 2025 Jun 20;137(1):4-22. doi: 10.1161/CIRCRESAHA.124.325750. Epub 2025 May 15. Circ Res. 2025. PMID: 40371535
-
Inflammatory Gene Expression of Human Perivascular Adipose Tissue in Abdominal Aortic Aneurysms.Eur J Vasc Endovasc Surg. 2021 Jun;61(6):1008-1016. doi: 10.1016/j.ejvs.2021.02.034. Epub 2021 Apr 12. Eur J Vasc Endovasc Surg. 2021. PMID: 33858751
-
Targeting vascular smooth muscle cell dysfunction with xanthine derivative KMUP-3 inhibits abdominal aortic aneurysm in mice.Atherosclerosis. 2020 Mar;297:16-24. doi: 10.1016/j.atherosclerosis.2020.01.029. Epub 2020 Feb 6. Atherosclerosis. 2020. PMID: 32059119
-
Relationships Between Perivascular Adipose Tissue and Abdominal Aortic Aneurysms.Front Endocrinol (Lausanne). 2021 Jun 14;12:704845. doi: 10.3389/fendo.2021.704845. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34194399 Free PMC article. Review.
-
Perivascular Adipose Tissue Regulates Vascular Function by Targeting Vascular Smooth Muscle Cells.Arterioscler Thromb Vasc Biol. 2020 May;40(5):1094-1109. doi: 10.1161/ATVBAHA.120.312464. Epub 2020 Mar 19. Arterioscler Thromb Vasc Biol. 2020. PMID: 32188271 Free PMC article. Review.
Cited by
-
The vascular microenvironment and its stem cells regulate vascular homeostasis.Front Cell Dev Biol. 2025 Mar 6;13:1544129. doi: 10.3389/fcell.2025.1544129. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40114970 Free PMC article. Review.
-
The role of long non-coding RNA in abdominal aortic aneurysm.Front Genet. 2023 Mar 15;14:1153899. doi: 10.3389/fgene.2023.1153899. eCollection 2023. Front Genet. 2023. PMID: 37007957 Free PMC article. Review.
-
Perivascular adipose tissue dysfunction contributes to thoracic aortic aneurysm development.Cardiovasc Diabetol. 2025 May 21;24(1):223. doi: 10.1186/s12933-025-02765-x. Cardiovasc Diabetol. 2025. PMID: 40399937 Free PMC article.
-
Anemoside B4 attenuates abdominal aortic aneurysm by limiting smooth muscle cell transdifferentiation and its mediated inflammation.Front Immunol. 2024 May 31;15:1412022. doi: 10.3389/fimmu.2024.1412022. eCollection 2024. Front Immunol. 2024. PMID: 38881898 Free PMC article.
-
Analysis of the relationship between central adiposity and biomechanical, histological, and immunohistochemical properties of the anterior wall of abdominal aortic aneurysms.JVS Vasc Sci. 2025 Feb 27;6:100283. doi: 10.1016/j.jvssci.2025.100283. eCollection 2025. JVS Vasc Sci. 2025. PMID: 40213095 Free PMC article.
References
-
- Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A. 1991;13:277–96.. - PubMed
-
- Verlohren S, Dubrovska G, Tsang SY, Essin K, Luft FC, Huang Y, et al. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension. 2004;44:271–6. doi: 10.1161/01.HYP.0000140058.28994.ec. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

