Respiratory indications for ECMO: focus on COVID-19
- PMID: 35945343
- PMCID: PMC9362963
- DOI: 10.1007/s00134-022-06815-w
Respiratory indications for ECMO: focus on COVID-19
Abstract
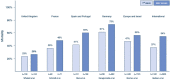
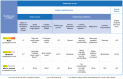
Extracorporeal membrane oxygenation (ECMO) is increasingly being used for patients with severe respiratory failure and has received particular attention during the coronavirus disease 2019 (COVID-19) pandemic. Evidence from two key randomized controlled trials, a subsequent post hoc Bayesian analysis, and meta-analyses support the interpretation of a benefit of ECMO in combination with ultra-lung-protective ventilation for select patients with very severe forms of acute respiratory distress syndrome (ARDS). During the pandemic, new evidence has emerged helping to better define the role of ECMO for patients with COVID-19. Results from large cohorts suggest outcomes during the first wave of the pandemic were similar to those in non-COVID-19 cohorts. As the pandemic continued, mortality of patients supported with ECMO has increased. However, the precise reasons for this observation are unclear. Known risk factors for mortality in COVID-19 and non-COVID-19 patients are higher patient age, concomitant extra-pulmonary organ failures or malignancies, prolonged mechanical ventilation before ECMO, less experienced treatment teams and lower ECMO caseloads in the treating center. ECMO is a high resource-dependent support option; therefore, it should be used judiciously, and its availability may need to be constrained when resources are scarce. More evidence from high-quality research is required to better define the role and limitations of ECMO in patients with severe COVID-19.
Keywords: ARDS; Acute respiratory distress syndrome; COVID-19; ECMO; Extracorporeal circulation; Extracorporeal membrane oxygenation; Resource limitations; Respiratory failure.
© 2022. Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
All authors have completed the ICMJE form (available upon request from the corresponding author). AS reports research grants and lecture fees from CytoSorbents and lecture fees from Abiomed, both outside the submitted work. EF received research support from Abbott and LivaNova and consulting fees from ALung Technologies, Vasomune, Baxter and GE Healthcare. CH is supported by an Australian NHMRC Investigator grant and leads the Australian and New Zealand ECMO Registry. CK received consulting fees from Xenios/ Fresenius and Bayer. He is Chair of the German ICU Registry and President of the German Society of Medical Intensive Care. JR reports lecture and advisory fees from Medtronic and Werfen, outside the submitted work. ASS has been on medical advisory boards for Baxter and Xenios; he is Chair of the Scientific Committee of the Committee of the International ECMO Network (ECMONet). DB receives research support from ALung Technologies. He has been on the medical advisory boards for Abiomed, Xenios, Medtronic, Inspira and Cellenkos. He is the President-elect of the Extracorporeal Life Support Organization (ELSO) and the Chair of ECMONet. All other authors report no conflicts of interest.
Figures

References
-
- Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D, collaboration Ct, Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

