Multiplatform molecular analyses refine classification of gliomas arising in patients with neurofibromatosis type 1
- PMID: 35945463
- PMCID: PMC9468105
- DOI: 10.1007/s00401-022-02478-5
Multiplatform molecular analyses refine classification of gliomas arising in patients with neurofibromatosis type 1
Abstract
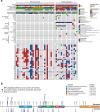
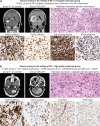
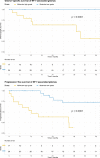
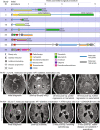
Gliomas arising in the setting of neurofibromatosis type 1 (NF1) are heterogeneous, occurring from childhood through adulthood, can be histologically low-grade or high-grade, and follow an indolent or aggressive clinical course. Comprehensive profiling of genetic alterations beyond NF1 inactivation and epigenetic classification of these tumors remain limited. Through next-generation sequencing, copy number analysis, and DNA methylation profiling of gliomas from 47 NF1 patients, we identified 2 molecular subgroups of NF1-associated gliomas. The first harbored biallelic NF1 inactivation only, occurred primarily during childhood, followed a more indolent clinical course, and had a unique epigenetic signature for which we propose the terminology "pilocytic astrocytoma, arising in the setting of NF1". The second subgroup harbored additional oncogenic alterations including CDKN2A homozygous deletion and ATRX mutation, occurred primarily during adulthood, followed a more aggressive clinical course, and was epigenetically diverse, with most tumors aligning with either high-grade astrocytoma with piloid features or various subclasses of IDH-wildtype glioblastoma. Several patients were treated with small molecule MEK inhibitors that resulted in stable disease or tumor regression when used as a single agent, but only in the context of those tumors with NF1 inactivation lacking additional oncogenic alterations. Together, these findings highlight recurrently altered pathways in NF1-associated gliomas and help inform targeted therapeutic strategies for this patient population.
Keywords: Astrocytoma; Brain tumor; Glioma; Molecular neuro-oncology; Molecular neuropathology; NF1; Neurofibromatosis type 1; Selumetinib; Trametinib.
© 2022. The Author(s).
Conflict of interest statement
A.P. and D.A.S. are members of the editorial board for
Figures



References
-
- Bajenaru ML, Hernandez MR, Perry A, Zhu Y, Parada LF, Garbow JR, Gutmann DH. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63:8573–8577. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

