Could widespread use of antiviral treatment curb the COVID-19 pandemic? A modeling study
- PMID: 35945513
- PMCID: PMC9361252
- DOI: 10.1186/s12879-022-07639-1
Could widespread use of antiviral treatment curb the COVID-19 pandemic? A modeling study
Abstract
Background: Despite the development of safe and effective vaccines, effective treatments for COVID-19 disease are still urgently needed. Several antiviral drugs have shown to be effective in reducing progression of COVID-19 disease.
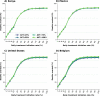
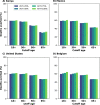
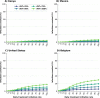
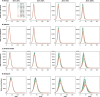
Methods: In the present work, we use an agent-based mathematical model to assess the potential population impact of the use of antiviral treatments in four countries with different demographic structure and current levels of vaccination coverage: Kenya, Mexico, United States (US) and Belgium. We analyzed antiviral effects on reducing hospitalization and death, and potential antiviral effects on reducing transmission. For each country, we varied daily treatment initiation rate (DTIR) and antiviral effect in reducing transmission (AVT).
Results: Irrespective of location and AVT, widespread antiviral treatment of symptomatic adult infections (20% DTIR) prevented the majority of COVID-19 deaths, and recruiting 6% of all adult symptomatic infections daily reduced mortality by over 20% in all countries. Furthermore, our model projected that targeting antiviral treatment to the oldest age group (65 years old and older, DTIR of 20%) can prevent over 30% of deaths. Our results suggest that early antiviral treatment (as soon as possible after inception of infection) is needed to mitigate transmission, preventing 50% more infections compared to late treatment (started 3 to 5 days after symptoms onset). Our results highlight the synergistic effect of vaccination and antiviral treatment: as the vaccination rate increases, antivirals have a larger relative impact on population transmission. Finally, our model projects that even in highly vaccinated populations, adding antiviral treatment can be extremely helpful to mitigate COVID-19 deaths.
Conclusions: These results suggest that antiviral treatments can become a strategic tool that, in combination with vaccination, can significantly reduce COVID-19 hospitalizations and deaths and can help control SARS-CoV-2 transmission.
Keywords: Agent-based model; Antiviral treatment; COVID-19; Mathematical model; SARS-CoV-2.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Update of
-
Could widespread use of antiviral treatment curb the COVID-19 pandemic? A modeling study.medRxiv [Preprint]. 2022 Apr 4:2021.11.10.21266139. doi: 10.1101/2021.11.10.21266139. medRxiv. 2022. Update in: BMC Infect Dis. 2022 Aug 9;22(1):683. doi: 10.1186/s12879-022-07639-1. PMID: 34790985 Free PMC article. Updated. Preprint.
References
-
- Johns Hopkins Coronavirus Resource Center. Home—Johns Hopkins Coronavirus Resource Center. 2021. https://coronavirus.jhu.edu/. Accessed 28 Sep 2021.
-
- Nicole B, Erika M. On behalf of the McGill University COVID19 Vaccine Tracker Team. COVID-19 vaccine development and approvals tracker. 2020. https://covid19.trackvaccines.org/. Accessed 28 Sep 2021.
-
- Campbell F, Archer B, Laurenson-Schafer H, Jinnai Y, Konings F, Batra N, Pavlin B, Vandemaele K, Van Kerkhove MD, Jombart T, Morgan O, le Polain de Waroux O. Increased transmissibility and global spread of sars-cov-2 variants of concern as at June 2021. Eurosurveillance 2021. 10.2807/1560-7917.ES.2021.26.24.2100509 - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R56AI143418/NH/NIH HHS/United States
- S10 OD028685/OD/NIH HHS/United States
- UM1 AI068635/NH/NIH HHS/United States
- R01CA152089/NH/NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- R01 CA152089/CA/NCI NIH HHS/United States
- S10OD028685/NH/NIH HHS/United States
- NU38OT000297-02/CC/CDC HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- UM1AI148684/NH/NIH HHS/United States
- UM1AI068619/NH/NIH HHS/United States
- P30AI050410/NH/NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- UM1 AI068617/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

