Aedes aegypti and Ae. albopictus microbiome/virome: new strategies for controlling arboviral transmission?
- PMID: 35945559
- PMCID: PMC9364528
- DOI: 10.1186/s13071-022-05401-9
Aedes aegypti and Ae. albopictus microbiome/virome: new strategies for controlling arboviral transmission?
Abstract
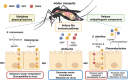
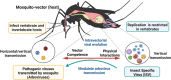
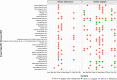
Aedes aegypti and Aedes albopictus are the main vectors of highly pathogenic viruses for humans, such as dengue (DENV), chikungunya (CHIKV), and Zika (ZIKV), which cause febrile, hemorrhagic, and neurological diseases and remain a major threat to global public health. The high ecological plasticity, opportunistic feeding patterns, and versatility in the use of urban and natural breeding sites of these vectors have favored their dispersal and adaptation in tropical, subtropical, and even temperate zones. Due to the lack of available treatments and vaccines, mosquito population control is the most effective way to prevent arboviral diseases. Resident microorganisms play a crucial role in host fitness by preventing or enhancing its vectorial ability to transmit viral pathogens. High-throughput sequencing and metagenomic analyses have advanced our understanding of the composition and functionality of the microbiota of Aedes spp. Interestingly, shotgun metagenomics studies have established that mosquito vectors harbor a highly conserved virome composed of insect-specific viruses (ISV). Although ISVs are not infectious to vertebrates, they can alter different phases of the arboviral cycle, interfering with transmission to the human host. Therefore, this review focuses on the description of Ae. aegypti and Ae. albopictus as vectors susceptible to infection by viral pathogens, highlighting the role of the microbiota-virome in vectorial competence and its potential in control strategies for new emerging and re-emerging arboviruses.
Keywords: Arbovirus; ISV; Metagenomics; Microbiota; Vectors; Virome.
© 2022. The Author(s).
Conflict of interest statement
All authors declare no conflict of interest.
Figures

Similar articles
-
Bidirectional Interactions between Arboviruses and the Bacterial and Viral Microbiota in Aedes aegypti and Culex quinquefasciatus.mBio. 2022 Oct 26;13(5):e0102122. doi: 10.1128/mbio.01021-22. Epub 2022 Sep 7. mBio. 2022. PMID: 36069449 Free PMC article.
-
Influence of dengue virus serotypes on the abundance of Aedes aegypti insect-specific viruses (ISVs).J Virol. 2024 Jan 23;98(1):e0150723. doi: 10.1128/jvi.01507-23. Epub 2023 Dec 14. J Virol. 2024. PMID: 38095414 Free PMC article.
-
Local-scale virome depiction in Medellín, Colombia, supports significant differences between Aedes aegypti and Aedes albopictus.PLoS One. 2022 Jul 27;17(7):e0263143. doi: 10.1371/journal.pone.0263143. eCollection 2022. PLoS One. 2022. PMID: 35895627 Free PMC article.
-
Minireview: Epidemiological impact of arboviral diseases in Latin American countries, arbovirus-vector interactions and control strategies.Pathog Dis. 2021 Sep 6;79(7):ftab043. doi: 10.1093/femspd/ftab043. Pathog Dis. 2021. PMID: 34410378 Review.
-
The virome of vector mosquitoes.Curr Opin Virol. 2021 Aug;49:7-12. doi: 10.1016/j.coviro.2021.04.002. Epub 2021 May 12. Curr Opin Virol. 2021. PMID: 33991759 Review.
Cited by
-
Multitarget Compounds for Neglected Diseases: A Review.Curr Drug Targets. 2024;25(9):577-601. doi: 10.2174/0113894501298864240627060247. Curr Drug Targets. 2024. PMID: 38967077 Review.
-
Is the Intergenic Region of Aedes aegypti Totivirus a Recombination Hotspot?Viruses. 2022 Nov 8;14(11):2467. doi: 10.3390/v14112467. Viruses. 2022. PMID: 36366565 Free PMC article.
-
Trends of Mansonia (Diptera, Culicidae, Mansoniini) in Porto Velho: Seasonal patterns and meteorological influences.PLoS One. 2024 May 8;19(5):e0303405. doi: 10.1371/journal.pone.0303405. eCollection 2024. PLoS One. 2024. PMID: 38718006 Free PMC article.
-
Role of the Microbiome in Aedes spp. Vector Competence: What Do We Know?Viruses. 2023 Mar 17;15(3):779. doi: 10.3390/v15030779. Viruses. 2023. PMID: 36992487 Free PMC article. Review.
-
RNA sequencing analysis of viromes of Aedes albopictus and Aedes vexans collected from NEON sites.Proc Natl Acad Sci U S A. 2025 May 20;122(20):e2403591122. doi: 10.1073/pnas.2403591122. Epub 2025 May 12. Proc Natl Acad Sci U S A. 2025. PMID: 40354533
References
-
- World Health Organization . Global vector control response 2017–2030. Geneva: World Health Organization; 2017.
-
- Mboera LE, Mweya CN, Rumisha SF, Tungu PK, Stanley G, Makange MR, Misinzo G, De Nardo P, Vairo F, Oriyo NM. The risk of dengue virus transmission in Dar es Salaam, Tanzania during an epidemic period of 2014. PLoS Negl Trop Dis. 2016;10:e0004313. doi: 10.1371/journal.pntd.0004313. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

