Tacrolimus induces a pro-fibrotic response in donor-derived human proximal tubule cells dependent on common variants of the CYP3A5 and ABCB1 genes
- PMID: 35945682
- PMCID: PMC9976759
- DOI: 10.1093/ndt/gfac237
Tacrolimus induces a pro-fibrotic response in donor-derived human proximal tubule cells dependent on common variants of the CYP3A5 and ABCB1 genes
Abstract
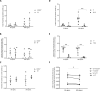
Background: Common genetic variants of the enzymes and efflux pump involved in tacrolimus disposition have been associated with calcineurin inhibitor nephrotoxicity, but their importance is unclear because of the multifactorial background of renal fibrosis. This study explores the pro-fibrotic response of tacrolimus exposure in relation to the differential capacity for tacrolimus metabolism in proximal tubule cells (PTCs) with a variable (pharmaco)genetic background.
Methods: PTCs were obtained from protocol allograft biopsies with different combinations of CYP3A5 and ABCB1 variants and were incubated with tacrolimus within the concentration range found in vivo. Gene and protein expression, CYP3A5 and P-glycoprotein function, and tacrolimus metabolites were measured in PTC. Connective tissue growth factor (CTGF) expression was assessed in protocol biopsies of kidney allograft recipients.
Results: PTCs produce CTGF in response to escalating tacrolimus exposure, which is approximately 2-fold higher in cells with the CYP3A5*1 and ABCB1 TT combination in vitro. Increasing tacrolimus exposure results in relative higher generation of the main tacrolimus metabolite {13-O-desmethyl tacrolimus [M1]} in cells with this same genetic background. Protocol biopsies show a larger increase in in vivo CTGF tissue expression over time in TT vs. CC/CT but was not affected by the CYP3A5 genotype.
Conclusions: Tacrolimus exposure induces a pro-fibrotic response in a PTC model in function of the donor pharmacogenetic background associated with tacrolimus metabolism. This finding provides a mechanistic insight into the nephrotoxicity associated with tacrolimus treatment and offers opportunities for a tailored immunosuppressive treatment.
Keywords: calcineurin inhibitor toxicity; connective tissue growth factor; genetic-dependent pro-fibrotic response; human donor proximal tubule cells; tacrolimus.
© The Author(s) 2022. Published by Oxford University Press on behalf of the ERA.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

