Environmental detection of Fasciola hepatica by loop-mediated isothermal amplification
- PMID: 35945935
- PMCID: PMC9357369
- DOI: 10.7717/peerj.13778
Environmental detection of Fasciola hepatica by loop-mediated isothermal amplification
Abstract
Fasciola hepatica, commonly referred to as liver flukes, is a substantial zoonotic parasitic disease of humans and livestock globally. While infection is readily controlled by anthelmintics, namely triclabendazole, the heavy reliance on triclabendazole has resulted in drug resistance appearing worldwide. Due to drug resistance, it is imperative to adopt an integrated parasite management program to preserve the efficacy of currently available anthelmintics. A integrated liver fluke management plan would benefit from a simple rapid, field-deployable diagnostic for detection of F. hepatica in environment and the host. Therefore, a rapid DNA test using loop-mediated isothermal amplification was developed and optimised for the detection of F. hepatica from faecal and water samples to enable the detection of parasites both within the host and from the environment. The assay presented here is fast, with amplification in ≤20 min, and highly sensitive, with a detection limit of 5 × 10-4 ng/µL. The workflow presented here provides a time to result of ≤60 min without requiring a commercial kit for the extraction of DNA from faecal and water samples, and pending further validation from field-samples, could potentially be used to enable real-time decision making to mitigate parasite prevalence on a farming property and with no requirement for sample transportation.
Keywords: Detection; Environment; Fasciola hepatica; Field; Fluke; LAMP; Parasite.
© 2022 Tran et al.
Conflict of interest statement
Travis Beddoe received funding from Cooperative Research Centre Project (CRC-P) awarded to Geneworks and La Trobe University, however, funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Figures
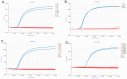
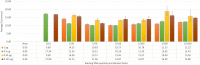

References
-
- Agriculture Victoria Diagnostic laboratory services price list-veterinary diagnostic services. DoJ, precincts and regions. 2019. https://agriculture.vic.gov.au/__data/assets/pdf_file/0010/536167/Price-... https://agriculture.vic.gov.au/__data/assets/pdf_file/0010/536167/Price-...
-
- Ai L, Dong SJ, Zhang WY, Elsheikha HM, Mahmmod YS, Lin RQ, Yuan ZG, Shi YL, Huang WY, Zhu XQ. Specific PCR-based assays for the identification of Fasciola species: their development, evaluation and potential usefulness in prevalence surveys. Annals of Tropical Medicine and Parasitology. 2010a;104(1):65–72. doi: 10.1179/136485910X12607012373713. - DOI - PubMed
-
- Ai L, Li C, Elsheikha HM, Hong SJ, Chen JX, Chen SH, Li X, Cai XQ, Chen MX, Zhu XQ. Rapid identification and differentiation of Fasciola hepatica and Fasciola gigantica by a loop-mediated isothermal amplification (LAMP) assay. Veterinary Parasitology. 2010b;174(3–4):228–233. doi: 10.1016/j.vetpar.2010.09.005. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

