Randomized feasibility trial of the Scleroderma Patient-centered Intervention Network hand exercise program (SPIN-HAND)
- PMID: 35945943
- PMCID: PMC9357372
- DOI: 10.7717/peerj.13471
Randomized feasibility trial of the Scleroderma Patient-centered Intervention Network hand exercise program (SPIN-HAND)
Abstract
Purpose: The Scleroderma Patient-centered Intervention Network (SPIN) online hand exercise program (SPIN-HAND), is an online self-help program of hand exercises designed to improve hand function for people with scleroderma. The objective of this feasibility trial was to evaluate aspects of feasibility for conducting a full-scale randomized controlled trial of the SPIN-HAND program.
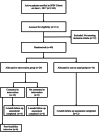
Materials and methods: The feasibility trial was embedded in the SPIN cohort and utilized the cohort multiple randomized controlled trial (cmRCT) design. In the cmRCT design, at the time of cohort enrollment, cohort participants consent to be assessed for trial eligibility and randomized prior to being informed about trials conducted using the cohort. When trials were conducted in the cohort, participants randomized to the intervention were informed and consented to access the intervention. Participants randomized to control were not informed that they have not received an intervention. All participants eligible and randomized to participate in the trial were included in analyses on an intent-to-treat basis. Cohort participants with a Cochin Hand Function Scale score ≥ 3/90 and an interest in using an online hand-exercise intervention were randomized (1:1 ratio) to be offered as usual care plus the SPIN-HAND Program or usual care for 3 months. User satisfaction was assessed with semi-structured interviews.
Results: Of the 40 randomized participants, 24 were allocated to SPIN-HAND and 16 to usual care. Of 24 participants randomized to be offered SPIN-HAND, 15 (63%) consented to use the program. Usage of SPIN-HAND content among the 15 participants who consented to use the program was low; only five (33%) logged in more than twice. Participants found the content relevant and easy to understand (satisfaction rating 8.5/10, N = 6). Automated eligibility and randomization procedures via the SPIN Cohort platform functioned properly. The required technical support was minimal.
Conclusions: Trial methodology functioned as designed, and the SPIN-HAND Program was feasibly delivered; however, the acceptance of the offer and use of program content among accepters were low. Adjustments to information provided to potential participants will be implemented in the full-scale SPIN-HAND trial to attempt to increase offer acceptance.
Trial registration: ClinicalTrials.gov NCT03092024.
Keywords: Cohort multiple RCT; Feasibility trial; Internet intervention; Occupational therapy; Physical therapy; Scleroderma; Systemic sclerosis; Tele-rehabilitation.
©2022 Kwakkenbos et al.
Conflict of interest statement
Luc Mouthon reported personal fees from Actelion/Johnson & Johnson, grants from LFB, non-financial support from Octapharma, and non-financial support from Grifols, all outside the submitted work. All other authors declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years. All authors declare no other relationships or activities that could appear to have influenced the submitted work.
Figures





References
-
- American College of Rheumatology Association of Rheumatology HealthProfessionals Interdisciplinary care for patients with rheumatic and musculoskeletal diseases by the rheumatology health care team. [19 October 2021]. https://www.rheumatology.org/Portals/0/Files/Multidisciplinary%20Care%20... https://www.rheumatology.org/Portals/0/Files/Multidisciplinary%20Care%20...
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

