Advances in delivery methods of Arthrospira platensis (spirulina) for enhanced therapeutic outcomes
- PMID: 35946342
- PMCID: PMC9373759
- DOI: 10.1080/21655979.2022.2100863
Advances in delivery methods of Arthrospira platensis (spirulina) for enhanced therapeutic outcomes
Abstract
Arthrospira platensis (A. platensis) aqueous extract has massive amounts of natural products that can be used as future drugs, such as C-phycocyanin, allophycocyanin, etc. This extract was chosen because of its high adaptability, which reflects its resolute genetic composition. The proactive roles of cyanobacteria, particularly in the medical field, have been discussed in this review, including the history, previous food and drug administration (FDA) reports, health benefits and the various dose-dependent therapeutic functions that A. platensis possesses, including its role in fighting against lethal diseases such as cancer, SARS-CoV-2/COVID-19, etc. However, the remedy will not present its maximal effect without the proper delivery to the targeted place for deposition. The goal of this research is to maximize the bioavailability and delivery efficiency of A. platensis constituents through selected sites for effective therapeutic outcomes. The solutions reviewed are mainly on parenteral and tablet formulations. Moreover, suggested enteric polymers were discussed with minor composition variations applied for better storage in high humid countries alongside minor variations in the polymer design were suggested to enhance the premature release hindrance of basic drugs in low pH environments. In addition, it will open doors for research in delivering active pharmaceutical ingredients (APIs) in femtoscale with the use of various existing and new formulations.Abbrevations: SDGs; Sustainable Development Goals, IL-4; Interleukin-4, HDL; High-Density Lipoprotein, LDL; Low-Density Lipoprotein, VLDL; Very Low-Density Lipoprotein, C-PC; C-Phycocyanin, APC; Allophycocyanin, PE; Phycoerythrin, COX-2; Cyclooxygenase-2, RCTs; Randomized Control Trials, TNF-α; Tumour Necrosis Factor-alpha, γ-LFA; Gamma-Linolenic Fatty Acid, PGs; Polyglycans, PUFAs: Polyunsaturated Fatty Acids, NK-cell; Natural Killer Cell, FDA; Food and Drug Administration, GRAS; Generally Recognized as Safe, SD; Standard Deviation, API; Active Pharmaceutical Ingredient, DW; Dry Weight, IM; Intramuscular, IV; Intravenous, ID; Intradermal, SC; Subcutaneous, AERs; Adverse Event Reports, DSI-EC; Dietary Supplement Information Executive Committee, cGMP; Current Good Manufacturing Process, A. platensis; Arthrospira platensis, A. maxima; Arthrospira maxima, Spirulina sp.; Spirulina species, Arthrospira; Spirulina, Tecuitlatl; Spirulina, CRC; Colorectal Cancer, HDI; Human Development Index, Tf; Transferrin, TfR; Transferrin Receptor, FR; Flow Rate, CPP; Cell Penetrating Peptide, SUV; Small Unilamenar Vesicle, LUV; Large Unilamenar Vesicle, GUV; Giant Unilamenar Vesicle, MLV; Multilamenar Vesicle, COVID-19; Coronavirus-19, PEGylated; Stealth, PEG; Polyethylene Glycol, OSCEs; Objective Structured Clinical Examinations, GI; Gastrointestinal Tract, CAP; Cellulose Acetate Phthalate, HPMCP, Hydroxypropyl Methyl-Cellulose Phthalate, SR; Sustained Release, DR; Delay Release, Poly(MA-EA); Polymethyl Acrylic Co-Ethyl Acrylate, f-DR L-30 D-55; Femto-Delay Release Methyl Acrylic Acid Co-Ethyl Acrylate Polymer, MW; Molecular Weight, Tg; Glass Transition Temperature, SN2; Nucleophilic Substitution 2, EPR; Enhance Permeability and Retention, VEGF; Vascular Endothelial Growth Factor, RGD; Arginine-Glycine-Aspartic Acid, VCAM-1; Vascular Cell Adhesion Molecule-1, P; Coefficient of Permeability, PES; Polyether Sulfone, pHe; Extracellular pH, ζ-potential; Zeta potential, NTA; Nanoparticle Tracking Analysis, PB; Phosphate Buffer, DLS; Dynamic Light Scattering, AFM; Atomic Force Microscope, Log P; Partition Coefficient, MR; Molar Refractivity, tPSA; Topological Polar Surface Area, C log P; Calculated Partition Coefficient, CMR; Calculated Molar Refractivity, Log S; Solubility Coefficient, pka; Acid Dissociation Constant, DDAB; Dimethyl Dioctadecyl Ammonium Bromide, DOPE; Dioleoylphosphatidylethanolamine, GDP; Good Distribution Practice, RES; Reticuloendothelial System, PKU; Phenylketonuria, MS; Multiple Sclerosis, SLE; Systemic Lupus Erythematous, NASA; National Aeronautics and Space Administration, DOX; Doxorubicin, ADRs; Adverse Drug Reactions, SVM; Support Vector Machine, MDA; Malondialdehyde, TBARS; Thiobarbituric Acid Reactive Substances, CRP; C-Reactive Protein, CK; Creatine Kinase, LDH; Lactated Dehydrogenase, T2D; Type 2 Diabetes, PCB; Phycocyanobilin, PBP; Phycobiliproteins, PEB; Phycoerythrobilin, DPP-4; Dipeptidyl Peptidase-4, MTT; 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide, IL-2; Interleukin-2, IL-6; Interleukin-6, PRISMA; Preferred Reporting Items for Systematic Reviews and Meta-Analyses, STATA; Statistics, HepG2; Hepatoblastoma, HCT116; Colon Cancer Carcinoma, Kasumi-1; Acute Leukaemia, K562; Chronic Leukaemia, Se-PC; Selenium-Phycocyanin, MCF-7; Breast Cancer Adenocarcinoma, A375; Human Melanoma, RAS; Renin-Angiotensin System, IQP; Ile-Gln-Pro, VEP; Val-Glu-Pro, Mpro; Main Protease, PLpro; Papin-Like Protease, BMI; Body Mass Index, IC50; Inhibitory Concentration by 50%, LD50; Lethal Dose by 50%, PC12 Adh; Rat Pheochromocytoma Cells, RNS; Reactive Nitrogen Species, Hb1Ac; hemoglobin A1c.
Keywords: Arthrospira maxima; Arthrospira platensis; controlled release; delay release; drug delivery; femtoscale; formulation development; liposomes; polymer optimization.
Plain language summary
Increase awareness of the impact and multi-disciplinary up-to-date roles of A. platensis on human lives and the importance of having further research on microalgae.Soliciting a critical analysis study on A. platensis biocomposition for drug delivery research.Insights on the correlation between ionization and drug bioavailability in specific sites in the human body.Offering solutions for improvising an optimized ‘Advanced Spirulina Dosage Forms’ products to maximize A. platensis therapeutic/pharmacological outcomes.Insights on existing biomaterials for optimization.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


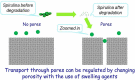

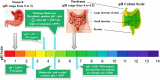
References
-
- Simonazzi A, Cid AG, Villegas M, et al. Nanotechnology applications in drug controlled release. Drug Target. Stimuli Sensitive Drug Deliv. Syst. 2018: 81–116. 10.1016/B978-0-12-813689-8.00003-3. - DOI
-
- Marc W. Harrold and Robin M. Zavod. Medicinal chemistry, chapter 2: functional group characteristics and roles. Basic Concepts Med Chem. 2020;21–66. 10.37573/9781585286027.002. - DOI
-
- Odiba A, Ukegbu C, Anunobi O, et al. Making drugs safer: improving drug delivery and reducing the side effect of drugs on the human biochemical system. Nanotechnol Rev. 2016;5(2). DOI: 10.1515/ntrev-2015-0055 - DOI
-
- Robinson SW, Afzal AM, Leader DP.. Bioinformatics: concepts, methods, and data. Med. 2014;259–287. DOI: 10.1016/B978-0-12-386882-4.00013-X - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
