Treatment patterns, healthcare resource utilization, and costs in patients with moderate-to-severe psoriasis treated with systemic therapy in Japan: A retrospective claims database study
- PMID: 35946343
- PMCID: PMC9804179
- DOI: 10.1111/1346-8138.16543
Treatment patterns, healthcare resource utilization, and costs in patients with moderate-to-severe psoriasis treated with systemic therapy in Japan: A retrospective claims database study
Abstract
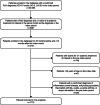
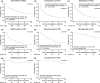
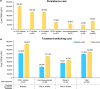
The real-world treatment landscape for patients with moderate-to-severe psoriasis receiving systemic therapies in Japan is not well understood. This study describes the demographic and clinical characteristics, treatment patterns, healthcare resource utilization, and psoriasis-associated costs in these patients. This retrospective observational study used data from the Japan Medical Data Center database between January 2016 and December 2020. Eligible patients had a confirmed diagnosis of psoriasis, ≥1 claim for a systemic treatment of interest, medical history for ≥6 months, and follow-up data for ≥12 months. Systemic therapies comprised biologics (tumor necrosis factor and interleukin inhibitors) and oral treatments (a phosphodiesterase-4 inhibitor, immunosuppressants, and vitamin A). Patient demographics and clinical characteristics, treatment patterns, healthcare resource utilization, and costs were evaluated. The study identified 1770 patients satisfying all inclusion criteria. The mean age was 49.0 years, with 68% of patients aged 20-54 years. Overall, 90.6% and 9.4% of patients received oral medications and biologics as index treatment, respectively. Treatment patterns, healthcare resource utilization, and costs were assessed for treatments received by ≥20 patients (n = 1730). During the 12-month follow-up period, 1102/1730 patients (63.7%) discontinued index treatment, of whom 9.9% switched to alternative systemic treatments. The persistence rate was ≥70% for most biologics and <50% for oral systemic treatments. All 1730 patients had ≥1 all-cause outpatient visit (2.0 visits per person per month) and hospitalization frequency was ≤0.01 per person per month. Persistent patients incurred inflation-adjusted costs of Japanese Yen (JPY) 88 667 per person per month. Treatment switching was associated with an increase in total cost: JPY 128 039 per person per month after switching versus JPY 117 504 before switching. This study of Japanese patients with moderate-to-severe psoriasis demonstrated low persistence, high discontinuation, and low rates of treatment switching with systemic therapies. Switching was associated with increased total cost. These results indicate unmet needs for new treatments.
Keywords: cost of illness; health resources; medication adherence; practice patterns; psoriasis.
© 2022 Bristol-Myers Squibb. The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Conflict of interest statement
YT reports grants from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Eisai, Eli Lilly, Kyowa Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe Pharma, Sun Pharmaceutical Industries Limited, Taiho Pharmaceutical, Torii Pharmaceutical, and UCB Pharma; consulting fees from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Janssen, Maruho, Novartis Pharma, Taiho Pharmaceutical, and UCB Pharma; and honoraria from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Eisai, Eli Lilly, Janssen, Kyowa Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe Pharma, Novartis Pharma, Sun Pharmaceutical Industries Limited, Taiho Pharmaceutical, Torii Pharmaceutical, and UCB Pharma. HK, KH, KT, YY, YZ, and YH are employees of and report profit of stock or stock options from Bristol Myers Squibb. DS is an employee of Bristol Myers Squibb. AM is an employee of Mu Sigma.
Figures
Similar articles
-
Real-world treatment patterns and healthcare costs among biologic-naive patients initiating apremilast or biologics for the treatment of psoriasis.J Med Econ. 2019 Apr;22(4):365-371. doi: 10.1080/13696998.2019.1571500. Epub 2019 Feb 4. J Med Econ. 2019. PMID: 30652520
-
Real-world psoriasis treatment patterns and disease burden in Germany, with a focus on biologics and apremilast: data from a German statutory health insurance database.J Med Econ. 2025 Dec;28(1):207-220. doi: 10.1080/13696998.2025.2452054. Epub 2025 Jan 21. J Med Econ. 2025. PMID: 39807542
-
Treatment patterns and healthcare resource utilization in palmoplantar pustulosis patients in Japan: A claims database study.PLoS One. 2020 May 22;15(5):e0232738. doi: 10.1371/journal.pone.0232738. eCollection 2020. PLoS One. 2020. PMID: 32442204 Free PMC article.
-
Adherence and resource use among psoriasis patients treated with biologics.Expert Rev Pharmacoecon Outcomes Res. 2018 Dec;18(6):609-617. doi: 10.1080/14737167.2018.1512408. Epub 2018 Sep 10. Expert Rev Pharmacoecon Outcomes Res. 2018. PMID: 30142007 Review.
-
Increasing Access to Effective Systemic Treatments in Patients with Moderate-to-Severe Psoriasis: Narrative Review.Dermatol Ther (Heidelb). 2023 Oct;13(10):2171-2185. doi: 10.1007/s13555-023-01014-x. Epub 2023 Sep 14. Dermatol Ther (Heidelb). 2023. PMID: 37710078 Free PMC article. Review.
Cited by
-
Healthcare Provider Administration of Biologics for Patients with Plaque Psoriasis: Literature Review and Clinical Considerations.J Clin Aesthet Dermatol. 2023 Dec;16(12 Suppl 2):S20-S25. J Clin Aesthet Dermatol. 2023. PMID: 38464741 Free PMC article. Review.
-
Real-World Discontinuation and Switching Patterns for Interleukin-Inhibitor Treatments in Patients with Moderate-to-Severe Psoriasis in Japan.Dermatol Ther (Heidelb). 2024 Jan;14(1):99-114. doi: 10.1007/s13555-023-01064-1. Epub 2023 Nov 29. Dermatol Ther (Heidelb). 2024. PMID: 38019410 Free PMC article.
-
Prevalence and incidence of comorbidities in patients with atopic dermatitis, psoriasis, alopecia areata, and vitiligo using a Japanese claims database.J Dermatol. 2025 May;52(5):841-854. doi: 10.1111/1346-8138.17643. Epub 2025 Feb 7. J Dermatol. 2025. PMID: 39921356 Free PMC article.
-
Factors Affecting Treatment Persistence in Japanese Patients with Psoriasis Prescribed Biologics: A Real-World Study Using an Insurance Claim Database.Dermatol Ther (Heidelb). 2024 Nov;14(11):2999-3015. doi: 10.1007/s13555-024-01274-1. Epub 2024 Oct 15. Dermatol Ther (Heidelb). 2024. PMID: 39407051 Free PMC article.
-
Deucravacitinib in plaque psoriasis: Safety and efficacy through 3 years in Japanese patients in the phase 3 POETYK PSO-1, PSO-4, and LTE trials.J Dermatol. 2025 May;52(5):761-772. doi: 10.1111/1346-8138.17685. Epub 2025 Mar 11. J Dermatol. 2025. PMID: 40066907 Free PMC article. Clinical Trial.
References
-
- Saeki H, Terui T, Morita A, Sano S, Imafuku S, Asahina A, et al. Japanese guidance for use of biologics for psoriasis (the 2019 version). J Dermatol. 2020;47:201–22. - PubMed
-
- Iizuka H. Pyramid plan for psoriasis treatment 2017. J Vis Dermatol. 2017;16:850–1.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

