Puerarin attenuates isoproterenol‑induced myocardial hypertrophy via inhibition of the Wnt/β‑catenin signaling pathway
- PMID: 35946454
- PMCID: PMC9437969
- DOI: 10.3892/mmr.2022.12822
Puerarin attenuates isoproterenol‑induced myocardial hypertrophy via inhibition of the Wnt/β‑catenin signaling pathway
Abstract
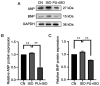
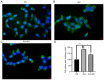
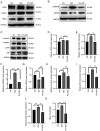
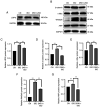
Myocardial hypertrophy (MH) is an independent risk factor for cardiovascular disease, which in turn lead to arrhythmia or heart failure. Therefore, attention must be paid to formulation of therapeutic strategies for MH. Puerarin is a key bioactive ingredient isolated from Pueraria genera of plants that is beneficial for the treatment of MH. However, its molecular mechanism of action has not been fully determined. In the present study, 40 µM puerarin was demonstrated to be a safe dose for human AC16 cells using Cell Counting Kit‑8 assay. The protective effects of puerarin against MH were demonstrated in AC16 cells stimulated with isoproterenol (ISO). These effects were characterized by a significant decrease in surface area of cells (assessed using fluorescence staining) and mRNA and protein expression levels of MH‑associated biomarkers, including atrial and brain natriuretic peptide, assessed using reverse transcription‑quantitative PCR and western blotting, as well as β‑myosin heavy chain mRNA expression levels. Mechanistically, western blotting demonstrated that puerarin inhibited activation of the Wnt signaling pathway. Puerarin also significantly decreased phosphorylation of p65; this was mediated via crosstalk between the Wnt and NF‑κB signaling pathways. An inhibitor (Dickkopf‑1) and activator (IM‑12) of the Wnt signaling pathway were used to demonstrate that puerarin‑mediated effects alleviated ISO‑induced MH via the Wnt signaling pathway. The results of the present study demonstrated that puerarin pre‑treatment may be a potential therapeutic strategy for preventing ISO‑induced MH and managing MH in the future.
Keywords: Wnt/β‑catenin signaling pathway; cardiac hypertrophy; isoproterenol; p65; phosphorylation of NF‑κB; puerarin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. - DOI - PMC - PubMed
-
- Cho YS, Moon SC, Ryu KS, Ryu KH. A study on clinical and healthcare recommending service based on cardiovascula disease pattern analysis. Int J Biosci Biotechnol. 2016;8:287–294.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

