Serum biomarkers in prednisolone-treated hand osteoarthritis patients
- PMID: 35946535
- PMCID: PMC9977113
- DOI: 10.1093/rheumatology/keac442
Serum biomarkers in prednisolone-treated hand osteoarthritis patients
Abstract
Objectives: To investigate whether biomarkers are modulated by prednisolone treatment in patients with hand OA and whether they can predict response to prednisolone.
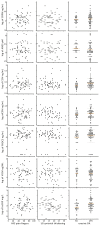
Methods: Biomarkers reflecting tissue turnover and inflammation [aggrecanase-derived neoepitope of arggecan (ARGS), MMP-derived neoepitope of type I collagen (C1M), MMP-derived neoepitope of type III collagen (C3M), marker of true type V collagen formation (PROC5), MMP-derived neoepitope of CRP (CRPM), citrullinated vimentin fragment (VICM), high-sensitivity (hsCRP)] were measured in sera from 78 patients with painful inflammatory hand OA, who were randomized between prednisolone or placebo treatment. Association of baseline biomarker levels with disease characteristics [visual analogue scale (VAS) pain, synovial thickening ultrasonography sum score and erosive OA] and OMERACT-Osteoarthritis Research Society International (OARSI) response after 6 weeks were analysed with linear or logistic regression and adjusted for age, BMI and sex. Change in biomarker levels after 6 weeks was assessed with linear regression adjusted for baseline biomarker levels, age, BMI and sex.
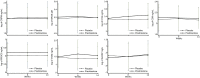
Results: For all patients (mean age 64 years, 79% female), there were no associations between biomarker levels and VAS finger pain or synovial thickening score at baseline. Patients with erosive hand OA had higher levels of C1M and hsCRP [adjusted geometric mean ratio 1.24 (95% CI 1.03, 1.49) and 1.91 (1.19, 3.06), respectively]. Biomarker levels did not decrease over time. There was no association between baseline biomarkers levels and OARSI response, except for CRPM [geometric mean ratio of 0.88 (0.77, 1.00)].
Conclusion: Erosive disease was associated with higher levels of C1M and hsCRP. Biomarker levels were not influenced by treatment with prednisolone. Current biomarkers were not associated with response to prednisolone in hand OA.
Keywords: RCT; biomarkers; hand osteoarthritis; prednisolone.
© The Author(s) 2022. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures
References
-
- Bay-Jensen AC, Thudium CS, Gualillo O, Mobasheri A.. Biochemical marker discovery, testing and evaluation for facilitating OA drug discovery and development. Drug Discov Today 2018;23:349–58. - PubMed
-
- Bay-Jensen AC, Manginelli AA, Karsdal M. et al. Low levels of type II collagen formation (PRO-C2) are associated with response to sprifermin: a pre-defined, exploratory biomarker analysis from the FORWARD study. Osteoarthritis Cartilage 2022;30:92–9. - PubMed
-
- Kroon FPB, Kortekaas MC, Boonen A. et al. Results of a 6-week treatment with 10 mg prednisolone in patients with hand osteoarthritis (HOPE): a double-blind, randomised, placebo-controlled trial. Lancet 2019;394:1993–2001. - PubMed
-
- Altman R, Alarcón G, Appelrouth D. et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum 1990;33:1601–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

