Collagen VI deficiency causes behavioral abnormalities and cortical dopaminergic dysfunction
- PMID: 35946603
- PMCID: PMC9548377
- DOI: 10.1242/dmm.049481
Collagen VI deficiency causes behavioral abnormalities and cortical dopaminergic dysfunction
Abstract
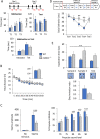
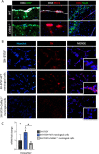
Mutations of genes coding for collagen VI (COL6) cause muscle diseases, including Ullrich congenital muscular dystrophy and Bethlem myopathy. Although COL6 genetic variants were recently linked to brain pathologies, the impact of COL6 deficiency in brain function is still largely unknown. Here, a thorough behavioral characterization of COL6-null (Col6a1-/-) mice unexpectedly revealed that COL6 deficiency leads to a significant impairment in sensorimotor gating and memory/attention functions. In keeping with these behavioral abnormalities, Col6a1-/- mice displayed alterations in dopaminergic signaling, primarily in the prefrontal cortex. In vitro co-culture of SH-SY5Y neural cells with primary meningeal fibroblasts from wild-type and Col6a1-/- mice confirmed a direct link between COL6 ablation and defective dopaminergic activity, through a mechanism involving the inability of meningeal cells to sustain dopaminergic differentiation. Finally, patients affected by COL6-related myopathies were evaluated with an ad hoc neuropsychological protocol, revealing distinctive defects in attentional control abilities. Altogether, these findings point towards a previously undescribed role for COL6 in the proper maintenance of dopamine circuitry function and its related neurobehavioral features in both mice and humans. This article has an associated First Person interview with the first author of the paper.
Keywords: Central nervous system; Cognitive function; Collagen VI; Dopamine; Mouse model; Prefrontal cortex.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



Similar articles
-
Collagen VI ablation in zebrafish causes neuromuscular defects during developmental and adult stages.Matrix Biol. 2022 Sep;112:39-61. doi: 10.1016/j.matbio.2022.08.004. Epub 2022 Aug 9. Matrix Biol. 2022. PMID: 35961424
-
Clinical and Molecular Spectrum Associated with COL6A3 c.7447A>G p.(Lys2483Glu) Variant: Elucidating its Role in Collagen VI-related Myopathies.J Neuromuscul Dis. 2021;8(4):633-645. doi: 10.3233/JND-200577. J Neuromuscul Dis. 2021. PMID: 33749658
-
Autophagy activation in COL6 myopathic patients by a low-protein-diet pilot trial.Autophagy. 2016 Dec;12(12):2484-2495. doi: 10.1080/15548627.2016.1231279. Epub 2016 Sep 22. Autophagy. 2016. PMID: 27656840 Free PMC article. Clinical Trial.
-
A novel de novo COL6A1 mutation emphasizes the role of intron 14 donor splice site defects as a cause of moderate-progressive form of ColVI myopathy - a case report and review of the genotype-phenotype correlation.Folia Neuropathol. 2017;55(3):214-220. doi: 10.5114/fn.2017.70486. Folia Neuropathol. 2017. PMID: 28984114 Review.
-
[Collagen VI-related muscle disorders].Brain Nerve. 2011 Nov;63(11):1169-78. Brain Nerve. 2011. PMID: 22068469 Review. Japanese.
Cited by
-
Collagen VI sustains cell stemness and chemotherapy resistance in glioblastoma.Cell Mol Life Sci. 2023 Jul 28;80(8):233. doi: 10.1007/s00018-023-04887-5. Cell Mol Life Sci. 2023. PMID: 37505240 Free PMC article.
-
Contextual fear memory impairment in Angelman syndrome model mice is associated with altered transcriptional responses.Sci Rep. 2023 Oct 30;13(1):18647. doi: 10.1038/s41598-023-45769-x. Sci Rep. 2023. PMID: 37903805 Free PMC article.
-
Stuck on you: Meninges cellular crosstalk in development.Curr Opin Neurobiol. 2023 Apr;79:102676. doi: 10.1016/j.conb.2023.102676. Epub 2023 Feb 9. Curr Opin Neurobiol. 2023. PMID: 36773497 Free PMC article. Review.
-
ADNP is essential for sex-dependent hippocampal neurogenesis, through male unfolded protein response and female mitochondrial gene regulation.Mol Psychiatry. 2025 Jun;30(6):2696-2706. doi: 10.1038/s41380-024-02879-w. Epub 2024 Dec 23. Mol Psychiatry. 2025. PMID: 39715923 Free PMC article.
-
Tic-related behaviors in Celsr3 mutant mice are contributed by alterations of striatal D3 dopamine receptors.Mol Psychiatry. 2025 Sep;30(9):3912-3924. doi: 10.1038/s41380-025-02970-w. Epub 2025 Mar 28. Mol Psychiatry. 2025. PMID: 40155412
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

