Spontaneous Decomposition of an Extraordinarily Twisted and Trans-Bent Fully-Phosphanyl-Substituted Digermene to an Unusual GeI Cluster
- PMID: 35946808
- PMCID: PMC9804623
- DOI: 10.1002/anie.202208851
Spontaneous Decomposition of an Extraordinarily Twisted and Trans-Bent Fully-Phosphanyl-Substituted Digermene to an Unusual GeI Cluster
Abstract
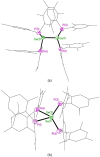
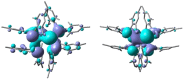
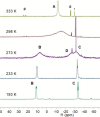
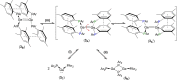
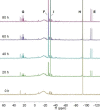
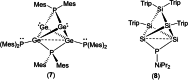
Ditetrelenes R2 E=ER2 (E=Si, Ge, Sn, Pb) substituted by multiple N/P/O/S-donor groups are extremely rare due to their propensity to disaggregate into their tetrylene monomers R2 E. We report the synthesis of the first fully phosphanyl-substituted digermene {(Mes)2 P}2 Ge=Ge{P(Mes)2 }2 (3, Mes=2,4,6-Me3 C6 H2 ), which adopts a highly unusual structure in the solid state, that is both strongly trans-bent and highly twisted. Variable-temperature 31 P{1 H} NMR spectroscopy suggests that 3 persists in solution, but is subject to a dynamic equilibrium between two conformations, which have different geometries about the Ge=Ge bond (twisted/non-twisted) due to a difference in the nature of their π-stacking interactions. Compound 3 undergoes unprecedented, spontaneous decomposition in solution to give a unique GeI cluster {(Mes)2 P}4 Ge4 ⋅5 CyMe (7).
Keywords: Cluster; Germanium; Multiple Bond; Phosphorus; Solid-State Structure.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no financial conflicts of interest.
Figures



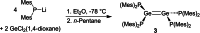
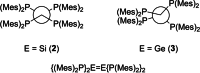
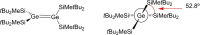


Similar articles
-
A Systematic Study of Structure and E-H Bond Activation Chemistry by Sterically Encumbered Germylene Complexes.Chemistry. 2016 Aug 8;22(33):11685-98. doi: 10.1002/chem.201601840. Epub 2016 Jul 6. Chemistry. 2016. PMID: 27381647
-
Synthesis and Reactivity Studies of Amido-Substituted Germanium(I)/Tin(I) Dimers and Clusters.Chemistry. 2019 Feb 21;25(11):2773-2785. doi: 10.1002/chem.201804770. Epub 2018 Dec 13. Chemistry. 2019. PMID: 30370947
-
P-Ge/Sn π Interactions Versus Arene···Ge/Sn Contacts for the Stabilization of Diphosphatetrylenes, (R2P)2E (E = Ge, Sn).Inorg Chem. 2020 Jan 6;59(1):863-874. doi: 10.1021/acs.inorgchem.9b03109. Epub 2019 Dec 19. Inorg Chem. 2020. PMID: 31855418
-
A Fully Phosphane-Substituted Disilene.Angew Chem Int Ed Engl. 2017 May 8;56(20):5593-5597. doi: 10.1002/anie.201701867. Epub 2017 Apr 12. Angew Chem Int Ed Engl. 2017. PMID: 28402025 Free PMC article.
-
Why do the heavy-atom analogues of acetylene E2H2 (E = Si-Pb) exhibit unusual structures?J Am Chem Soc. 2005 May 4;127(17):6290-9. doi: 10.1021/ja042295c. J Am Chem Soc. 2005. PMID: 15853336
Cited by
-
Polymorphism in a secondary phosphine.Acta Crystallogr C Struct Chem. 2025 Feb 1;81(Pt 2):109-113. doi: 10.1107/S2053229625000555. Epub 2025 Jan 30. Acta Crystallogr C Struct Chem. 2025. PMID: 39907072 Free PMC article.
References
-
- None
-
- Lenoir D., Smith P. J., Liebman J. F., Nicolaides A., Johnson R. P., Konrad K. M., Strained Hydrocarbons (Ed.: Dodziuk H.), Wiley-VCH, Weinheim, 2009;
-
- Wiberg K. B., Reactive Intermediate Chemistry (Eds.: Moss R. A., Platz M. S., Jones, Jr. M.), Wiley, Hoboken, 2004, pp. 717–740;
-
- Takezawa H., Murase T., Fujita M., J. Am. Chem. Soc. 2012, 134, 17420–17423; - PubMed
-
- ter Wiel M. K. J., Kwit M. G., Meetsma A., Feringa B. L., Org. Biomol. Chem. 2007, 5, 87–96. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

