Comparing the Efficacy and Safety of Gemcitabine plus Nab-Paclitaxel versus Gemcitabine Alone in Older Adults with Unresectable Pancreatic Cancer
- PMID: 35946841
- PMCID: PMC9526497
- DOI: 10.1093/oncolo/oyac157
Comparing the Efficacy and Safety of Gemcitabine plus Nab-Paclitaxel versus Gemcitabine Alone in Older Adults with Unresectable Pancreatic Cancer
Abstract
Background: Gemcitabine plus nab-paclitaxel (GnP) has been a standard treatment for unresectable pancreatic cancer (uPC); however, the current treatment status and usefulness in older adults with uPC remain unclear. Therefore, we aimed to investigate the patient background and compare the efficacy and safety of GnP versus other treatments in older adults with uPC.
Patients and methods: In this prospective observational study, we enrolled 233 eligible patients aged ≥76 years with pathologically proven, clinically uPC, and no history of chemotherapy from 55 Japanese centers during September 2018-September 2019. The main endpoints were overall survival (OS), progression-free survival (PFS), and safety. Geriatric assessments were performed upon registration and after 3 months. To adjust for confounders, we conducted propensity score-matched analyses.
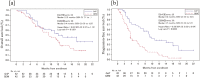
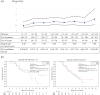
Results: GnP, gemcitabine alone (Gem), best supportive care, and other therapies were administered to 116, 72, 16, and 29 patients, respectively. In the propensity score-matched analysis, 42 patients each were selected from the GnP and Gem groups. The median OS was longer in the GnP group than in the Gem group (12.2 vs. 9.4 months; hazard ratio [HR], 0.65; 95% CI, 0.37-1.13). The median PFS was significantly longer in the GnP group than in the Gem group (9.2 vs. 3.7 months; HR, 0.38; 95% CI, 0.23-0.64). The incidence of severe adverse events was higher with GnP than with Gem; however, the difference was not significant.
Conclusion: GnP is more efficacious than Gem in patients aged ≥76 years with uPC despite demonstrating a higher incidence of severe adverse events.
Keywords: aged; albumin-bound paclitaxel; gemcitabine; geriatric assessment; pancreatic cancer; vulnerable population.
© The Author(s) 2022. Published by Oxford University Press.
Figures

References
-
- International Agency for Research on Cancer. GLOBOCAN , https://gco.iarc.fr/; 2021. [accessed on May 24, 2021].
-
- Cancer Information Service, National Cancer Centre. Vital Statistics in Japan , https://ganjoho.jp/en/professional/statistics/table_download.html [accessed on June 28, 2021].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

