Tocolytics for delaying preterm birth: a network meta-analysis (0924)
- PMID: 35947046
- PMCID: PMC9364967
- DOI: 10.1002/14651858.CD014978.pub2
Tocolytics for delaying preterm birth: a network meta-analysis (0924)
Abstract
Background: Preterm birth is the leading cause of death in newborns and children. Tocolytic drugs aim to delay preterm birth by suppressing uterine contractions to allow time for administration of corticosteroids for fetal lung maturation, magnesium sulphate for neuroprotection, and transport to a facility with appropriate neonatal care facilities. However, there is still uncertainty about their effectiveness and safety.
Objectives: To estimate relative effectiveness and safety profiles for different classes of tocolytic drugs for delaying preterm birth, and provide rankings of the available drugs.
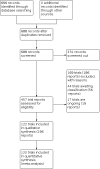
Search methods: We searched Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov (21 April 2021) and reference lists of retrieved studies.
Selection criteria: We included all randomised controlled trials assessing effectiveness or adverse effects of tocolytic drugs for delaying preterm birth. We excluded quasi- and non-randomised trials. We evaluated all studies against predefined criteria to judge their trustworthiness.
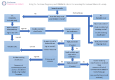
Data collection and analysis: At least two review authors independently assessed the trials for inclusion and risk of bias, and extracted data. We performed pairwise and network meta-analyses, to determine the relative effects and rankings of all available tocolytics. We used GRADE to rate the certainty of the network meta-analysis effect estimates for each tocolytic versus placebo or no treatment.
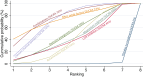
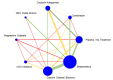
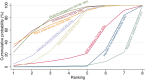
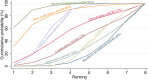
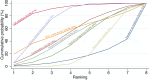
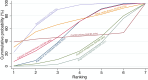
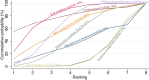
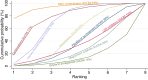
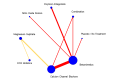
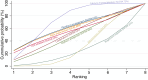
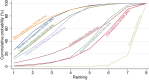
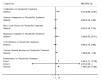
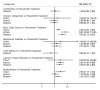
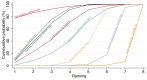
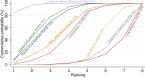
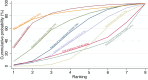
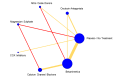
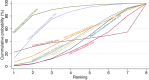
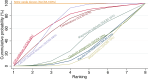
Main results: This network meta-analysis includes 122 trials (13,697 women) involving six tocolytic classes, combinations of tocolytics, and placebo or no treatment. Most trials included women with threatened preterm birth, singleton pregnancy, from 24 to 34 weeks of gestation. We judged 25 (20%) studies to be at low risk of bias. Overall, certainty in the evidence varied. Relative effects from network meta-analysis suggested that all tocolytics are probably effective in delaying preterm birth compared with placebo or no tocolytic treatment. Betamimetics are possibly effective in delaying preterm birth by 48 hours (risk ratio (RR) 1.12, 95% confidence interval (CI) 1.05 to 1.20; low-certainty evidence), and 7 days (RR 1.14, 95% CI 1.03 to 1.25; low-certainty evidence). COX inhibitors are possibly effective in delaying preterm birth by 48 hours (RR 1.11, 95% CI 1.01 to 1.23; low-certainty evidence). Calcium channel blockers are possibly effective in delaying preterm birth by 48 hours (RR 1.16, 95% CI 1.07 to 1.24; low-certainty evidence), probably effective in delaying preterm birth by 7 days (RR 1.15, 95% CI 1.04 to 1.27; moderate-certainty evidence), and prolong pregnancy by 5 days (0.1 more to 9.2 more; high-certainty evidence). Magnesium sulphate is probably effective in delaying preterm birth by 48 hours (RR 1.12, 95% CI 1.02 to 1.23; moderate-certainty evidence). Oxytocin receptor antagonists are probably effective in delaying preterm birth by 48 hours (RR 1.13, 95% CI 1.05 to 1.22; moderate-certainty evidence), are effective in delaying preterm birth by 7 days (RR 1.18, 95% CI 1.07 to 1.30; high-certainty evidence), and possibly prolong pregnancy by 10 days (95% CI 2.3 more to 16.7 more). Nitric oxide donors are probably effective in delaying preterm birth by 48 hours (RR 1.17, 95% CI 1.05 to 1.31; moderate-certainty evidence), and 7 days (RR 1.18, 95% CI 1.02 to 1.37; moderate-certainty evidence). Combinations of tocolytics are probably effective in delaying preterm birth by 48 hours (RR 1.17, 95% CI 1.07 to 1.27; moderate-certainty evidence), and 7 days (RR 1.19, 95% CI 1.05 to 1.34; moderate-certainty evidence). Nitric oxide donors ranked highest for delaying preterm birth by 48 hours and 7 days, and delay in birth (continuous outcome), followed by calcium channel blockers, oxytocin receptor antagonists and combinations of tocolytics. Betamimetics (RR 14.4, 95% CI 6.11 to 34.1; moderate-certainty evidence), calcium channel blockers (RR 2.96, 95% CI 1.23 to 7.11; moderate-certainty evidence), magnesium sulphate (RR 3.90, 95% CI 1.09 to 13.93; moderate-certainty evidence) and combinations of tocolytics (RR 6.87, 95% CI 2.08 to 22.7; low-certainty evidence) are probably more likely to result in cessation of treatment. Calcium channel blockers possibly reduce the risk of neurodevelopmental morbidity (RR 0.51, 95% CI 0.30 to 0.85; low-certainty evidence), and respiratory morbidity (RR 0.68, 95% CI 0.53 to 0.88; low-certainty evidence), and result in fewer neonates with birthweight less than 2000 g (RR 0.49, 95% CI 0.28 to 0.87; low-certainty evidence). Nitric oxide donors possibly result in neonates with higher birthweight (mean difference (MD) 425.53 g more, 95% CI 224.32 more to 626.74 more; low-certainty evidence), fewer neonates with birthweight less than 2500 g (RR 0.40, 95% CI 0.24 to 0.69; low-certainty evidence), and more advanced gestational age (MD 1.35 weeks more, 95% CI 0.37 more to 2.32 more; low-certainty evidence). Combinations of tocolytics possibly result in fewer neonates with birthweight less than 2500 g (RR 0.74, 95% CI 0.59 to 0.93; low-certainty evidence). In terms of maternal adverse effects, betamimetics probably cause dyspnoea (RR 12.09, 95% CI 4.66 to 31.39; moderate-certainty evidence), palpitations (RR 7.39, 95% CI 3.83 to 14.24; moderate-certainty evidence), vomiting (RR 1.91, 95% CI 1.25 to 2.91; moderate-certainty evidence), possibly headache (RR 1.91, 95% CI 1.07 to 3.42; low-certainty evidence) and tachycardia (RR 3.01, 95% CI 1.17 to 7.71; low-certainty evidence) compared with placebo or no treatment. COX inhibitors possibly cause vomiting (RR 2.54, 95% CI 1.18 to 5.48; low-certainty evidence). Calcium channel blockers (RR 2.59, 95% CI 1.39 to 4.83; low-certainty evidence), and nitric oxide donors probably cause headache (RR 4.20, 95% CI 2.13 to 8.25; moderate-certainty evidence).
Authors' conclusions: Compared with placebo or no tocolytic treatment, all tocolytic drug classes that we assessed (betamimetics, calcium channel blockers, magnesium sulphate, oxytocin receptor antagonists, nitric oxide donors) and their combinations were probably or possibly effective in delaying preterm birth for 48 hours, and 7 days. Tocolytic drugs were associated with a range of adverse effects (from minor to potentially severe) compared with placebo or no tocolytic treatment, although betamimetics and combination tocolytics were more likely to result in cessation of treatment. The effects of tocolytic use on neonatal outcomes such as neonatal and perinatal mortality, and on safety outcomes such as maternal and neonatal infection were uncertain.
Trial registration: ClinicalTrials.gov NCT01429545 NCT00306462 NCT02132533 NCT00811057 NCT00185900 NCT00599898 NCT02538718 NCT00185952 NCT00116623 NCT00463736 NCT00525486 NCT00620724 NCT00641784 NCT01314859 NCT01360034 NCT01577121 NCT01796522 NCT01985594 NCT02438371 NCT02583633 NCT03040752 NCT00486824 NCT03369262 NCT03976063 NCT00466128 NCT01869361 NCT02725736 NCT03129945 NCT03298191 NCT03542552 NCT04404686 NCT04846621.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
This project was supported by the National Institute for Health Research, via ESP Incentive Award Scheme funding to Cochrane Pregnancy and Childbirth (award number NIHR150766).
Ioannis D Gallos: The World Health Organization provided payment to Ioannis Gallos for working on this review. Ioannis is a health professional at Birmingham Women's Hosptital. Ioannis is an Associate Editor for Cochrane Pregnancy and Childbirth, but had no involvement in the editorial processing of this review. Ioannis was also awarded an NIHR ESP incentive award for completion of this review (NIHR150766).
Amie Wilson: works as a Midwife at Birmingham Women's and Children's Hospital Foundation Trusth, and has no declarations of interest.
Victoria A Hodgetts‐Morton: works as a NIHR clinical lecturer in O&G at the University of Birmingham and Birmingham Women's Hospital. Victoria reports personally receiving funds from Hologic, LLC as an Independent Contractor.
Ella Marson: has no declarations of interest.
Alexandra Markland: has no declarations of interest.
Eva Larkai: has no declarations of interest.
Argyro Papadopoulou: is currently a PhD student at the University of Birmingham, UK. Her tuition fees are paid by Tommy's charity, Tommy's National Centre for Miscarriage Research. Tuition fees are directly paid to the University of Birmingham. Argyro works as a Resident at Alexandra University Hosptial, Athens, Greece.
Arri Coomarasamy: has no declarations of interest.
Aurelio Tobias: has no declarations of interest.
Doris Chou: in terms of guideline and recommendation synthesis, I manage the maternal/perinatal living guideline process within the World Health Organization. The technical group may consider this review in deliberations related to the use of tocolytics. During these meetings, I do not carry any voting capacity.
Olufemi T Oladapo: is an Editor with Cochrane Pregnancy and Childbirth, but had no involvement with the editorial processing of this review.
Malcolm J Price: has no declarations of interest.
Katie Morris: has acted as an Independent Contractor for the British Maternal and Fetal Medicine Society, NHS England, Royal College of Obstetricians and Gynaecologists and Tommy's Baby Charity and did not receive funds personally for this work. Kate has also acted as an Independent Contractor for Surepulse and received consultant fees personally for this work. Her institution received funds for a National Institute for Health Research grant, which she held. Kate has published several invited reviews and book chapters related to preterm birth and works as a Consultant in Maternal Fetal Medicine at Birmingham Womens and Childrens Hospital NHS Foundation Trust.
Figures


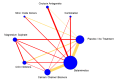
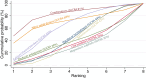
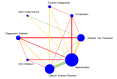


















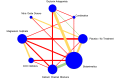
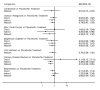

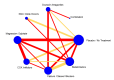
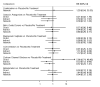








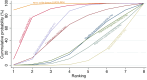
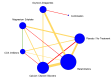


































































































































































































































































































































































































































































































































































































































































































































































































































































































Update of
References
References to studies included in this review
Adam 1966 {published data only}
-
- Adam GS. Isoxuprine and premature labour. Australian & New Zealand Journal of Obstetrics & Gynaecology 1966;6:294-8. - PubMed
Ally 1992 {published data only}
-
- Ally K, Nicolas A, Thoumsin H, Lambotte R. Magnesium gluconate and intravenous tocolysis with ritodrine. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1992;21(4):370-4. - PubMed
Al Omari 2013 {published data only}
-
- Al-Omari WR, Begam MA, Khan FS, Khudhair IY, Nagelkerke NJ. Single versus combination therapy in acute tocolysis: a prospective randomized controlled trial. Open Journal of Obstetrics and Gynecology 2013;3:249-56.
-
- NCT01429545. Single versus combination therapy in acute tocolysis. https://clinicaltrials.gov/show/NCT01429545 (first received 07 September 2011).
Al Qattan 2000 {published data only}
-
- Al-Qattan F, Omu AE, Labeeb N. A prospective randomized study comparing nifedipine versus ritodrine for the suppression of preterm labour. Medical Principles and Practice 2000;9(3):164-73. [DOI: 10.1159/000054241] - DOI
Amorim 2009 {published data only}
-
- Amorim MM, Lippo LA, Costa AA, Coutinho IC, Souza AS. Transdermal nitroglycerin versus oral nifedipine administration for tocolysis: a randomized clinical trial. Revista Brasileira de Ginecologia e Obstetricia 2009;31(11):552-8. - PubMed
Ara 2008 {published data only}
-
- Ara I, Banu H. A prospective randomised trial of nifedipine versus placebo in preterm labour. Bangladesh Journal of Obstetrics and Gynecology 2008;23(2):61-4.
Aramayo 1990 {published data only}
-
- Aramayo JF, Martinez FJ, Rosales CL. Tocolytic therapy with magnesium sulphate and terbutaline for inhibition of pre-term labor. Ginecologia y Obstetricia de Mexico 1990;58:265-9. - PubMed
Asgharnia 2002 {published data only}
-
- Asgharnia M, Sobhani A, Omidvar-Jalali Z. Comparison of Mg-sulfate and indomethacin in management of women with preterm labor. Journal of Gorgan University of Medical Sciences 2002;4(10):7-12.
Beall 1985 {published data only}
-
- Beall MH, Edgar BW, Paul RH, Smith-Wallace T. A comparison of ritodrine, terbutaline, and magnesium sulfate for the suppression of preterm labor. American Journal of Obstetrics and Gynecology 1985;153:854-9. - PubMed
Besinger 1991 {published data only}
Bisits 1998 {published data only}
-
- Bisits A, Madsen G, McLean M, O'Callaghan S, Smith R, Giles W. Corticotropin-releasing hormone: a biochemical predictor of preterm delivery in a pilot randomized trial of the treatment of preterm labor. American Journal of Obstetrics and Gynecology 1998;178(4):862-6. [DOI: 10.1016/s0002-9378(98)60503-2] - DOI - PubMed
Bisits 2004 {published data only}
-
- Giles W, Knox M, Madsen G, Bisits A, Gill A, Smith R, et al. The randomised nitric oxide tocolysis trial. American Journal of Obstetrics and Gynecology 2001;184(1):S6. - PubMed
-
- Gill A, Giles W, Bisits A, Madsen G, Knox M, Tudehope D, et al. Neonatal neurodevelopmental outcomes following tocolysis with glycerol trinitrate patches and IV beta 2 agonist therapy. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S117. - PubMed
Borna 2007 {published data only}
Bracero 1991 {published data only}
-
- Bracero LA, Leikin E, Kirshenbaum N, Tejani N. Comparison of nifedipine and ritodrine for the treatment of preterm labor. In: 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23-27; Houston, Texas, USA. 1990:77. - PubMed
Cabar 2008 {published data only}
-
- Cabar FR, Bittar RE, Gomes CM, Zugab M. Atosiban as a tocolytic agent: a new proposal of a therapeutic approach. Revista Brasileira de Ginecologia y Obstetricia 2008;30(2):87-92. - PubMed
Canadian Preterm Labor Investigators 1992 {published data only}
Cararach 2006 {published data only}
-
- Cararach V, Palacio M, Martinez S, Deulofeu P, Sanchez M, Cobo T, et al. Nifedipine versus ritodrine for suppression of preterm labor. Comparison of their efficacy and secondary effects. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2006;127(2):204-8. [DOI: 10.1016/j.ejogrb.2005.10.020] - DOI - PubMed
Christensen 1980 {published data only}
-
- Christensen KK, Ingemarsson I, Leideman T, Solum T, Svenningsen N. Effect of ritodrine on labor after premature rupture of the membranes. Obstetrics and Gynecology 1980;55(2):187-90. - PubMed
Colon 2016 {published data only}
-
- Colon I, Berletti M, Garabedian MJ, Wilcox N, Williams K, El-Sayed YY, et al. Randomized, double-blinded trial of magnesium sulfate tocolysis versus intravenous normal saline for preterm nonsevere placental abruption. American Journal of Obstetrics and Gynecology 2016;212(1 Suppl 1):S388-S389. [DOI: 10.1055/s-0036-1571324] - DOI - PubMed
-
- Colon I, Berletti M, Garabedian MJ, Wilcox N, Williams K, El-Sayed YY, et al. Randomized, double-blinded trial of magnesium sulfate tocolysis versus intravenous normal saline for preterm nonsevere placental abruption. American Journal of Perinatology 2016;33(7):696-702. [DOI: 10.1055/s-0036-1571324] - DOI - PubMed
Cotton 1984 {published data only}
-
- Cotton DB, Strassner HT, Hill LM, Schifrin BS, Paul RH. Comparison of magnesium sulfate, terbutaline and a placebo for inhibition of preterm labor. A randomized study. Journal of Reproductive Medicine 1984;29(2):92-7. - PubMed
Cox 1990 {published data only}
-
- Cox SM, Sherman ML, Leveno KJ. Single-center randomized trial of magnesium sulfate for inhibition of uterine contractions in preterm labor. In: 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23-27; Houston, Texas, USA. 1990:39.
de Heus 2009 {published data only}
Ehsanipoor 2011 {published data only}
-
- Ehsanipoor RM, Shrivastava VK, Lee RM, Chan K, Galyean AM, Garite TJ, et al. A randomized, double-masked trial of prophylactic indomethacin tocolysis versus placebo in women with premature rupture of membranes. American Journal of Perinatology 2011;28(6):473-8. [DOI: 10.1055/s-0030-1270118] - DOI - PubMed
El Sayed 1999 {published data only}
European Atosiban Study 2001 {published data only}
-
- European Atosiban Study Group. The oxytocin antagonist atosiban versus the beta-agonist terbutaline in the treatment of preterm labor. A randomized, double-blind, controlled study. Acta Obstetricia et Gynecologica Scandinavica 2001;80(5):413-22. - PubMed
Ferguson 1984 {published data only}
Ferguson 1990 {published data only}
Floyd 1992 {published data only}
-
- Floyd RC, McLaughlin BN, Martin RW, Roberts WE, Wiser WL, Morrison JC. Comparison of magnesium and nifedipine for primary tocolysis and idiopathic preterm labor. American Journal of Obstetrics and Gynecology 1992;166:446.
-
- Floyd RC, McLauglin BN, Perry KG Jr, Martin RW, Sullivan CA, Morrison JC. Magnesium sulfate or nifedipine hydrochloride for acute tocolysis of preterm labor: efficacy and side effects. Journal of Maternal-fetal Investigation 1995;5(1):25-9.
Fox 1993 {published data only}
-
- Fox MD, Allbert JR, McCaul JF, Martin RW, McLaughlin BN, Morrison JC. Neonatal morbidity between 34 and 37 weeks' gestation. Journal of Perinatology 1993;XIII(5):349-53. - PubMed
Francioli 1988 {published data only}
-
- Francioli M, De Meuron A. Usefulness of the addition of aspartate magnesium hydrochloride via intravenous route to beta mimetics in the treatment of threatened premature labor. Revue Medicale de la Suisse Romande 1988;108:283-9. - PubMed
French and Australian Atosiban Investigators 2001 {published data only}
-
- French/Australian Atosiban Investigators Group. Treatment of preterm labor with the oxytocin antagonist atosiban: a double-blind, randomized, controlled comparison with salbutamol. European journal of Obstetrics, Gynecology, and Reproductive Biology 2001;98(2):177-85. [DOI: 10.1016/s0301-2115(01)00331-1] - DOI - PubMed
-
- The Worldwide Atosiban versus Beta-antagonists Study Group. Effectiveness and safety of the oxytocin antagonist atosiban versus beta-adrenergic agonists in the treatment of preterm labour. BJOG: an international journal of obstetrics and gynecology 2001;108(2):133-42. - PubMed
Gamissans 1982 {published data only}
-
- Gamissans O, Cararach V, Serra, J. The role of prostaglandin-inhibitors, beta-adrenergic drugs and glucocorticoids in the management of threatened preterm labor. In: Jung H, Lamberti G , editors(s). Beta-mimetic Drugs in Obstetrics and Perinatology. 3rd Symposium on Beta-mimetic Drugs; 1980 Nov; Aachen. Stuttgart: Georg Thieme, 1982:71-84.
Ganla 1999 {published data only}
-
- Ganla KM, Shroff SA, DesaiI S, Bhinde AG. A prospective comparison of nifedipine and isoxsuprine for tocolysis. Bombay Hospital journal 1999;41(2):259-63.
Garcia‐Velasco 1998 {published data only}
Garite 1987 {published data only}
-
- Garite TJ, Keegan KA, Freeman RK, Nageotte MP. A randomized trial of ritodrine tocolysis versus expectant management in patients with premature rupture of membranes at 25 to 30 weeks of gestation. American Journal of Obstetrics and Gynecology 1987;157(2):388-93. [DOI: 10.1016/s0002-9378(87)80179-5] - DOI - PubMed
George 1991 {published data only}
-
- George SS, George K, Jairaj P. A randomized controlled study of nifedipine and isoxuprine in the treatment of preterm labor. Journal of Obstetrics and Gynaecology of India 1991;41(6):765-67.
Glock 1993 {published data only}
-
- Morales WJ, Glock JL. Efficacy and safety of nifedipine vs magnesium sulfate in the management of preterm labor: a randomized study. American Journal of Obstetrics and Gynecology 1993;168:375 [SPO Abstract 119]. - PubMed
Goodwin 1994 {published data only}
-
- Goodwin TM, Paul RH, Silver H, Parsons M, Chez R, Spellacy W, et al. Safety and efficacy of the oxytocin antagonist atosiban in threatened preterm labor: initial US trial. American Journal of Obstetrics and Gynecology 1992;166:359. - PubMed
Goodwin 1996 {published data only}
Guinn 1997 {published data only}
-
- Guinn DA, Goepfert AR, Owen J, Brumfield CG, Hauth JC. Management options in women with preterm uterine contractions: a randomized clinical trial. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S44. - PubMed
Haghighi 1999 {published data only}
-
- NCT00306462. Trial of magnesium sulfate tocolysis versus nifedipine tocolysis in women with preterm labor. https://clinicaltrials.gov/show/NCT00306462 (first received 23 March 2006).
Haghighi 2005 {published data only}
Hatjis 1987 {published data only}
-
- Hatjis CG, Swain M, Nelson LH, Meis PJ, Ernest JM. Efficacy of combined administration of magnesium sulfate and ritodrine in the treatment of premature labor. Obstetrics and Gynecology 1987;69(3 Pt 1):317-22. - PubMed
Hawkins 2019 {published data only}
-
- Hawkins JS, Wells CE, Casey BM, McIntire DD, Leveno KJ. Nifedipine for acute tocolysis of preterm labor: a placebo-controlled randomized trial. Obstetrics and Gynecology 2021;138(1):73-8. - PubMed
-
- NCT02132533. Nifedipine for acute tocolysis of preterm labor. https://clinicaltrials.gov/show/NCT02132533 (first received 7 May 2014).
He 2002 {published data only}
-
- He Q, Sha J, Gu Q, Gu H, Chen X, Yang Z, et al. Clinical effect and mechanism of nitroglycerin patch on arresting preterm labor. Zhonghua Fu Chan Ke za Zhi 2002;37(3):134-5. - PubMed
Hollander 1987 {published data only}
How 1998 {published data only}
-
- How H, Cook C, Cook V, Spinnato J. Preterm premature rupture of membranes: aggressive tocolysis versus expectant management. American Journal of Obstetrics and Gynecology 1996;174:306. - PubMed
How 2006 {published data only}
-
- How H, Zafaranchi L, Stella C, Recht K, Maxwell R, Sibai B, et al. Magnesium sulfate (MGSO4) tocolysis versus no tocolysis in women with preterm labor between 32 0/7 and 34 6/7 weeks of gestation: a randomized controlled trial [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S6. - PubMed
-
- How HY, Zafaranchi L, Stella CL, Recht K, Maxwell RA, Sibai BM, et al. Tocolysis in women with preterm labor between 32 0/7 and 34 6/7 weeks of gestation: a randomized controlled pilot study. American Journal of Obstetrics and Gynecology 2006;194(4):976-81. [DOI: 10.1016/j.ajog.2006.02.030] - DOI - PubMed
Howard 1982 {published data only}
-
- Howard TE, Killam AP, Penney LL, Daniell WC. A double blind randomized study of terbutaline in premature labor. Military Medicine 1982;147(4):305-7. - PubMed
Ingemarsson 1976 {published data only}
Jaju 2011 {published data only}
Janky 1990 {published data only}
-
- Janky E, Leng JJ, Cormier P, Salamon R, Meynard J. A randomised study of treatment of threatened premature labour: nifedipine as against ritodrine. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1990;19:478-82. - PubMed
Jannet 1997 {published data only}
-
- Jannet D, Abankwa A, Guyard B, Carbonne B, Marpeau L, Milliez J. Nicardipine versus salbutamol in the treatment of premature labor. A prospective randomized study. European Journal of Obstetrics, Gynecology, and Reproductive biology 1997;73(1):11-6. [DOI: 10.1016/s0301-2115(97)02701-2] - DOI - PubMed
Kara 2009 {published data only}
-
- Kara M, Yilmaz E, Avci I, Oge T. Comparison of nifedipine with magnesium sulphate plus terbutaline for the treatment of preterm labor. Turk Jinekoloji Ve Obstetrik Dernegi Dergisi 2009;6(4):250-6.
Kashanian 2005 {published data only}
Kashanian 2011 {published data only}
-
- IRCT138901312624N. A comparison of the 2 methods for the treatment of preterm labor. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT138901312624N5.
-
- Zolali B. A comparison between nifedipin and indomethacin for the treatment of preterm labor and their side effects. Journal of Maternal & Neonatal Medicine 2014;27:363. [DOI: ]
Kashanian 2014 {published data only}
-
- IRCT201108262624N. Treatment of preterm labor. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201108262624N8.
-
- Kashanian M, Zamen Z, Khorshidifar A. A comparison of effectiveness between skin patch of nitroglycerin and nifedipin on controlling preterm labor. Razi Journal of Medical Sciences 2013;19(103):26-32.
-
- Kashanian M. Comparison between nitroglycerin dermal patch and nifedipine for treatment of preterm labor. International Journal of Gynecology and Obstetrics 2018;143 Suppl 3:168. [DOI: 10.1002/ijgo.12582] - DOI
Kashanian 2020 {published data only}
-
- IRCT20091023002624N. Treatment of preterm labor. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20091023002624N26 (first received 2018).
-
- Kashanian M, Shirvani S, Sheikhansari N, Javanmanesh F. A comparative study on the efficacy of nifedipine and indomethacin for prevention of preterm birth as monotherapy and combination therapy: a randomized clinical trial. Journal of Maternal-fetal & Neonatal Medicine 2020;33(19):3215-20. [DOI: 10.1080/14767058.2019.1570117] - DOI - PubMed
Klauser 2014 {published data only}
-
- Klauser CK, Briery CM, Tucker AR, Martin RW, Magann EF, Chauhan SP, et al. Tocolysis in women with advanced preterm labor: a secondary analysis of a randomized clinical trial. Journal of Maternal-fetal & Neonatal Medicine 2016;29(5):696-700. [DOI: ] - PubMed
-
- NCT00811057. Tocolysis for preterm labor. https://clinicaltrials.gov/show/NCT00811057 (first received 18 December 2008).
Koks 1998 {published data only}
Kose 1995 {published data only}
-
- Kose D, Karaosmanoglu S, Yeniguc CT, Yucesoy I, Ozben C, Baysal C. Efficacy and safety of nifedipin in the management of preterm labor. Jinekoloji Ve Obstetrik Dergisi 1995;9:165-70.
Kramer 1999 {published data only}
-
- Kramer W, Saade G, Belfort M, Dorman K, Mayes M, Moyes K. Randomized double blind study comparing sulindac to terbutaline: fetal cardiovascular effects. American Journal of Obstetrics and Gynecology 1996;174(1 Pt 2):326. - PubMed
-
- Kramer WB, Saade GR, Belfort M, Dorman K, Mayes M, Moise KJ Jr. A randomized double-blind study comparing the fetal effects of sulindac to terbutaline during the management of preterm labor. American Journal of Obstetrics and Gynecology 1999;180(2 Pt 1):396-401. [DOI: 10.1016/s0002-9378(99)70221-8] - DOI - PubMed
Kupferminc 1993 {published data only}
-
- Kupferminc M, Lessing JB, Peyser MR. A comparative, prospective, randomized study of nifedipine vs ritodrine for suppressing preterm labor. In: 39th Annual Meeting of the Society for Gynecologic Investigation; 1992 March 18-21; San Antonio, Texas, USA. 1992:335.
Kurki 1991b {published data only}
-
- Eronen M, Pesonen E, Kurki T, Ylikorkala O, Hallman M. The effects of indomethacin and beta-sympathomimetic agent on the fetal ductus arteriosus during treatment of premature labor: a randomized double-blind study. American Journal of Obstetrics and Gynecology 1991;164(1 Pt 1):141-6. [DOI: 10.1016/0002-9378(91)90644-7] - DOI - PubMed
-
- Kurki T, Eronen M, Lumme R, Ylikorkala O. A randomized double-dummy comparison between indomethacin and nylidrin in threatened preterm labor. Obstetrics and Gynecology 1991;78(6):1093-7. - PubMed
-
- Kurki T, Laatikainen T, Salminen-Lappalainen K, Ylikorkala O. Maternal plasma corticotrophin-releasing hormone - elevated in preterm labour but unaffected by indomethacin or nylidrin. British Journal of Obstetrics and Gynaecology 1991;98(7):685-91. - PubMed
Laohapojanart 2007 {published data only}
-
- Laohapojanart N, Soorapan S, Wacharaprechanont T, Ratanajamit C. Safety and efficacy of oral nifedipine versus terbutaline injection in preterm labor. Chotmaihet Thangphaet [Journal of the Medical Association of Thailand] 2007;90(11):2461-9. - PubMed
Larmon 1999 {published data only}
-
- Ross EL, Ross BS, Dickerson GA, Fischer RG, Morrison JC. Oral nicardipine versus intravenous magnesium sulfate for the treatment of preterm labor. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):181. - PubMed
Larsen 1980 {published data only}
-
- Hesseldahl H. A Danish multicenter study on ritodrine R in the treatment of pre-term labour. Danish Medical Bulletin 1979;26:116-8. - PubMed
-
- Kristoffersen K, Hansen MK. The condition of the foetus and infant in cases treated with ritodrine R. Danish Medical Bulletin 1979;26(3):121-2. - PubMed
-
- Larsen JF, Hansen MK, Hesseldahl H, Kristoffersen K, Larsen PK, Osler M, et al. Ritodrine in the treatment of preterm labour. A clinical trial to compare a standard treatment with three regimens involving the use of ritodrine. British Journal of Obstetrics and Gynaecology 1980;87(11):949-57. [DOI: 10.1111/j.1471-0528.1980.tb04457.x] - DOI - PubMed
Larsen 1986 {published data only}
Leake 1983 {published data only}
-
- Leake RD, Hobel CJ, Okada DM, Ross MG, Williams PR. Neonatal metabolic effects of oral ritodrine hydrochloride administration. Pediatric Pharmacology (New York, N.Y.) 1983;3(2):101-6. - PubMed
Lees 1999 {published data only}
Leveno 1986 {published data only}
-
- Leveno KJ, Klein VR, Guzick DS, Williams ML, Young DC, Hankins G. A single-center randomized, controlled trial of ritodrine hydrochloride. In: 6th Annual Meeting of the Society of Perinatal Obstetricians; 1986 Jan 30-Feb 1; San Antonio, Texas, USA. 1986:155.
Lin 2009 {published data only}
-
- Lin CH, Lin SY, Shyu MK, Chen SU, Lee CN. Randomized trial of oxytocin antagonist atosiban versus beta-adrenergic agonists in the treatment of spontaneous preterm labor in Taiwanese women. Journal of the Formosan Medical Association / Taiwan yi zhi 2009;108(6):493-501. [DOI: 10.1016/S0929-6646(09)60097-8] - DOI - PubMed
Lyell 2007a {published data only}
-
- Lyell D, Pullen K, Campbell L, Ching S, Burrs D, Chitkara U, et al. Magnesium sulfate versus nifedipine for acute tocolysis of preterm labor [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S18. - PubMed
-
- NCT00185900. Magnesium sulfate versus nifedipine for the acute tocolysis of preterm labor: a prospective, randomized trial. https://clinicaltrials.gov/show/NCT00185900 (first received 16 September 2005).
Matsuda 1993 {published data only}
-
- Matsuda Y, Ikenoue T, Ibara, S, Sameshima H, Kuraya, K, Hokanishi H. The efficacy of prophylactic antibiotic and tocolytic therapy for premature rupture of the membranes--a prospective randomized study. Nihon Sanka Fujinka Gakkai Zasshi 1993;45(10):1109-114. - PubMed
Mawaldi 2008 {published data only}
McWhorter 2004 {published data only}
-
- McWhorter J, Carlan SJ, O Leary TD. Rofecoxib versus magnesium sulfate to arrest preterm labor: a randomized double-blind trial. Obstetrics and Gynecology 2002;99(4 Suppl):2S. - PubMed
Meyer 1990 {published data only}
-
- Meyer WR, Randall HW, Graves WL. Nifedipine vs ritodrine for suppressing preterm labor. Journal of Reproductive Medicine 1990;35:649-53. - PubMed
Miller 1982 {published data only}
-
- Miller JM, Keane MW, Horger EO. Comparison of magnesium sulfate and terbutaline for the arrest of premature labor. Journal of Reproductive Medicine 1982;27(6):348-51. - PubMed
Morales 1989 {published data only}
-
- Morales WJ, Smith SG, Angel JL, O'Brien WF, Knuppel RA. Efficacy and safety of indomethacin versus ritodrine in the management of preterm labor: a randomized study. Obstetrics and Gynecology 1989;74(4):567-72. - PubMed
-
- Morales WJ, Smith SG, Angel JL, O'Brien WF, Knuppel RA. Efficacy and safety of indomethacin vs ritodrine in the management of preterm labor: a randomized study. In: 9th Annual Meeting of the Society of Perinatal Obstetricians; 1989 Feb 1-4; New Orleans, Louisiana, USA. 1989:35.
Moutquin 2000 {published data only}
-
- Moutquin JM, Sherman D, Cohen H, Mohide PT, Hochner-Celnikier D, Fejgin M, et al. Double-blind, randomized, controlled trial of atosiban and ritodrine in the treatment of preterm labor: a multicenter effectiveness and safety study. American Journal of Obstetrics and Gynecology 2000;182(5):1191-9. [DOI: 10.1067/mob.2000.104950] - DOI - PubMed
Neri 2009 {published data only}
Niebyl 1980 {published data only}
-
- Blake DA, Niebyl JR, White RD, Kumor KM, Dubin NH, Robinson JC, et al. Treatment of premature labor with indomethacin. Advances in Prostaglandin and Thromboxane Research 1980;8:1465-7. - PubMed
Nijman 2016 {published data only}
-
- EUCTR2011-000174-66-NL. Assessment of perinatal outcome by use of tocolysis in early labour (APOSTEL IV). http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2011-000174-66-NL (first received 2012).
-
- Nijman TA, Van Vliet EO, Naaktgeboren CA, Oude Rengerink K, De Lange TS, Bax CJ, et al. Nifedipine versus placebo in the treatment of preterm prelabor rupture of membranes: a randomized controlled trial: assessment of perinatal outcome by use of tocolysis in early labor-APOSTEL IV trial. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2016;205:79-84. [DOI: 10.1016/j.ejogrb.2016.08.024] - DOI - PubMed
-
- NTR3363. Assessment of perinatal outcome by use of tocolysis in early labour: nifedipine versus placebo in the treatment of preterm premature rupture of membranes. http://www.who.int/trialsearch/Trial2.aspx?TrialID=NTR3363.
-
- Oudijk M, Nijman TA, Van Vliet EO, Rengerink KO, De Lange T, Bax CJ, et al. Nifedipine versus placebo in the treatment of preterm premature rupture of membranes. Assessment of perinatal outcome by use of tocolysis in early labor - APOSTEL IV study. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S62, Abstract no: 88. - PubMed
Nonnenmacher 2009 {published data only}
Padovani 2015 {published data only}
-
- Padovani TR, Lopes LC. Nifedipine and terbutaline: comparative study of effectiveness and safety in preventing preterm labor. International journal of Gynecology and Obstetrics 2012;119 Suppl 3:S761. [DOI: 10.1016/S0020-7292%2812%2961899-2] - DOI
Papatsonis 1997 {published data only}
-
- Papatsonis DN, Van Geijn HP, Bleker OP, Lange FM, Ader HJ, Dekker GA. Tocolytic efficacy of nifedipine versus ritodrine; results of a randomized trial. American Journal of Obstetrics and Gynecology 1996;174:306.
Parilla 1997 {published data only}
Parsons 1987 {published data only}
-
- Parsons MT, Owens CA, Spellacy WN. Thermic effects of tocolytic agents: decreased temperature with magnesium sulfate. Obstetrics and Gynecology 1987;69(1):88-90. - PubMed
Pezzati 2001 {published data only}
-
- Pezzati M, Giani T, Gambi B, Dani C, Bertini G, Biagiotti R, et al. Influence of maternal magnesium sulphate and ritodrine treatment on cerebral blood flow velocity of the preterm newborn. Acta Obstetricia et Gynecologica Scandinavica 2001;80:818-23. - PubMed
Raymajhi 2003 {published data only}
-
- Raymajhi R, Pratap K. A comparative study between nifedipine and isoxsuprine in the suppression of preterm labour. Kathmandu University Medical Journal (KUMJ) 2003;1(2):85-90. - PubMed
Read 1986 {published data only}
Richter 2005 {published data only}
Romero 2000 {published data only}
-
- Romero R, Sibai BM, Sanchez-Ramos L, Valenzuela GJ, Veille JC, Tabor B, et al. An oxytocin receptor antagonist (atosiban) in the treatment of preterm labor: a randomized, double-blind, placebo-controlled trial with tocolytic rescue. American Journal of Obstetrics and Gynecology 2000;182(5):1173-83. [DOI: 10.1067/mob.2000.95834] - DOI - PubMed
-
- Sibai BM, Romero R, Sanchex-Ramos L, Valenzuela G, Veille JC, Tabor B, et al. A double-blind placebo-controlled trial of an oxytocin-receptor antagonist (antocin) in the treatment of preterm labor. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S2. - PubMed
Saade 2021 {published data only}
-
- EUCTR2014-003326-41-GB. Randomized, double-blind, multicenter, phase III study comparing the efficacy and safety of retosiban versus placebo for women in spontaneous Preterm labor - NEWBORN-1. https://trialsearch.who.int/?TrialID=EUCTR2014-003326-41-IT (first received 2015).
-
- NCT02377466. A phase III efficacy and safety study of intravenous retosiban versus placebo for women in spontaneous preterm labor. https://clinicaltrials.gov/show/NCT02377466 (first received 26 February 2015).
Sakamoto 1985 {published data only}
-
- Sakamoto S. Effectiveness of oral ritodrine hydrochloride on preventing tocolysis: a multicentre double-blinded trial. Igaku No Ayumi 1985;133(10):734-51.
Salim 2012 {published data only}
-
- NCT00599898. Nifedipine compared to atosiban for treating preterm labor. https://clinicaltrials.gov/ct2/show/NCT00599898 (first received 8 January 2008).
-
- Salim R, Garmi G, Nachum Z, Zafran N, Baram S, Shalev E. Nifedipine compared with atosiban for treating preterm labor: a randomized controlled trial. Obstetrics and Gynecology 2012;120(6):1323-31. [DOI: ] - PubMed
Schleussner 2003 {published data only}
-
- Schleussner E, Moller A, Gross W, Kahler C, Moller U, Richter S, et al. Maternal and fetal side effects of tocolysis using transdermal nitroglycerin or intravenous fenoterol combined with magnesium sulfate. European Journal of Obstetrics, Gynecology, and Reproductive biology 2003;106(1):14-9. [DOI: 10.1016/s0301-2115(02)00197-5] - DOI - PubMed
Schorr 1998 {published data only}
Shim 2006 {published data only}
-
- Shim JY, Park YW, Yoon BH, Cho YK, Yang JH, Lee Y, et al. Multicentre, parallel group, randomised, single-blind study of the safety and efficacy of atosiban versus ritodrine in the treatment of acute preterm labour in Korean women. BJOG: an international journal of obstetrics and gynaecology 2006;113(11):1228-34. [DOI: 10.1111/j.1471-0528.2006.01053.x] - DOI - PubMed
Smith 1999 {published data only}
-
- Smith GN, Walker MC, McGrath MJ. Randomized double-blind, placebo controlled trial assessing nitroglycerin as a tocolytic. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S181.
Smith 2007 {published data only}ISRCTN20129681
-
- ISRCTN20129681. The Canadian preterm labour nitroglycerin trial. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN20129681 (first received 2005 ).
-
- Smith G, Walker M, Ohlsson A, O'Brien K, Rory W. Transdermal nitroglycerin for preterm labor. In: Pediatric Academic Societies Annual Meeting; 2006 April 29-May 2; San Francisco, CA, USA. 2006.
-
- Smith G [personal communication]. Canadian preterm labour nitroglycerin trial. Letter to K Duckitt (with a copy to Cochrane Pregnancy and Childbirth, Liverpool, UK) 13 June 2002.
Spellacy 1979 {published data only}
-
- Spellacy WN, Cruz AC, Birk SA, Buhi WC. Treatment of premature labour with ritodrine: a randomized controlled study. Obstetrics and Gynecology 1979;54:220-3. - PubMed
Surichamorn 2001 {published data only}
-
- Surichamorn P. The efficacy of terbutaline and magnesium sulfate in the management of preterm labor. Chotmaihet Thangphaet [Journal of the Medical Association of Thailand] 2001;84(1):98-104. - PubMed
Szulc 2000 {published data only}
-
- Szulc E, Leibschang J. Comparative evaluation of efficiency and tolerance of two alternative methods for premature uterine contractions suppression using fenoterol and nitroglycerin. Medycyna Wieku Rozwojowego 2000;4(3):307-16. - PubMed
Taherian 2006 {published data only}
-
- Taherian AA, Dehdar P. Comparison of efficacy and safety of nifedipine versus magnesium sulfate in treatment of preterm labor. Journal of Research in Medical Sciences 2007;12(3):136-42.
Tchilinguirian 1984 {published data only}
-
- Tchilinguirian NG, Najem R, Sullivan GB, Craparo FJ. The use of ritodrine and magnesium sulfate in the arrest of premature labor. International Journal of Gynecology and Obstetrics 1984;22:117-23. - PubMed
Thornton 2009 {published data only}
-
- NCT00209326. A proof of concept study assessing the effect of four different single bolus intravenous doses of FE200440 and placebo on stopping preterm labor. https://clinicaltrials.gov/show/NCT00209326 (first received 12 September 2005).
-
- Thornton S, Goodwin TM, Greisen G, Hedegaard M, Arce JC. The effect of a selective oxytocin antagonist (barusiban) in threatened preterm labour: a randomized, double-blind, placebo-controlled trial. In: 55th Annual Meeting of the Society of Gynecologic Investigation; 2008 March 26-29; San Diego, USA. 2008:Abstract no: 129.
-
- Thornton S, Goodwin TM, Greisen G, Hedegaard M, Arce JC. The effect of barusiban on plasma concentrations and uterine contractility in threatened preterm labour: a randomised, double-blind, placebo-controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93 Suppl 1:Fa11-Fa12.
-
- Thornton S, Goodwin TM, Greisen G, Hedegaard MA, Arce JC. The effect of barusiban, a selective oxytocin antagonist, in threatened preterm labor at late gestational age: a randomized, double-blind, placebo-controlled trial. American Journal of Obstetrics and Gynecology 2009;200(6):627.e1-627.e10. [DOI: 10.1016/j.ajog.2009.01.015] - DOI - PubMed
Thornton 2015 {published data only}
-
- Snidow J, Miller H, Valenzuela G, Thornton S, Stier B, Clayton L, et al. A multicenter, randomized, double-blind, placebo-controlled phase 2 trial of retosiban, a selective oxytocin receptor antagonist, for the management of preterm labor. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl 1):S155.
Tohoku 1984 {published data only}
-
- Tohoku Research Group for Prevention of Preterm Birth. Effectiveness of ritodrine hydrochloride for tocolysis in threatened preterm delivery - double blinded trial. Igaku No Ayumi 1984;131(4):270-8.
Trabelsi 2008 {published data only}
-
- Trabelsi K, Hadj Taib H, Amouri H, Abdennadheur W, Ben Amar H, Kallel W, et al. Nicardipine versus salbutamol in the treatment of premature labor: comparison of their efficacy and side effects. Tunisie Medicale 2008;86(1):43-8. - PubMed
Valdes 2012 {published data only}
Van De Water 2008 {published data only}
Van Vliet 2016 {published data only}
-
- EUCTR2009-015782-30-NL. The effectiveness of tocolytic agents in the improvement of neonatal outcome in women with threatened preterm labour. http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2009-015782-30-NL (first received 2012).
-
- Mol BW, Vliet EO, Oudijk MA. Nifedipine versus atosiban for tocolysis in preterm labour (assessment of perinatal outcome after specific tocolysis in early labour: APOSTEL III-trial). Journal of Paediatrics and Child Health 2015;51 Suppl 1:35. [DOI: ]
-
- NTR2947. Nifedipine versus Atosiban in the treatment of threatened preterm labour: APOSTEL III. https://www.trialregister.nl/trial/2806 (first received 6 January 2014). - PMC - PubMed
-
- Van Vliet E, Schuit E, Heida K, Kok M, Gyselaers W, Porath M, et al. Nifedipine versus atosiban for tocolysis in preterm labour (Assessement of Perinatal Outcome after Specific Tocolysis in Early Labour: APOSTEL III-trial). American Journal of Obstetrics and Gynecology 2015;212(1 Suppl 1):S54.
Vis 2014 {published data only}
-
- Vis JY, Van Baaren GJ, Wilms FF, Oudijk MA, Kwee A, Porath MM, et al. Randomized comparison of nifedipine and placebo in fibronectin-negative women with symptoms of preterm labor and a short cervix (APOSTEL-I Trial). American Journal of Perinatology 2015;32(5):451-60. [DOI: 10.1055/s-0034-1390346] - DOI - PubMed
Walters 1977 {published data only}
Wang 2000 {published data only}
-
- Wang H, Zeng W, Liu H, Ou Y. A randomized controlled trial on the treatment of preterm labor with ritodrine hydrochloride and magnesium sulfate. Hua Xi Yi Ke da Xue Xue Bao [Journal of West China University of Medical Sciences] 2000;31(4):515-7.
Wani 2004 {published data only}
Weerakul 2002 {published data only}
Wilkins 1988 {published data only}
Zhang 2002 {published data only}
-
- Zhang X, Liu M. Clinical observations on the prevention and treatment of premature labor with nifedipine. Hua-hsi i Ko Ta Hsueh Hsueh Pao [Journal of West China University of Medical Sciences] 2002;33(2):288-90. - PubMed
Zhu 1996 {published data only}
-
- Zhu B, Fu Y. Treatment of preterm labor with ritodrine. Zhonghua Fu Chan Ke za Zhi 1996;31(12):721-3. - PubMed
Zuckerman 1984 {published data only}
-
- Zuckerman H, Shalev E, Gilad G, Katzuni E. Further study of the inhibition of premature labor by indomethacin. Part II. Double-blind study. Journal of Perinatal Medicine 1984;12(1):25-9. - PubMed
References to studies excluded from this review
ACTRN12616000748415 {published data only}
-
- ACTRN12616000748415. Comparative study between Nifedipine, progesterone and ritodrine for maintenance tocolysis in management of preterm labour. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12616000748415 (first received 2016).
ACTRN12617001639314 {published data only}
-
- ACTRN12617001639314. A randomised controlled trial of sulindac to delay premature birth in pregnancies complicated by a short cervix. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12617001639314 (first received 2017).
Alavi 2015a {published data only}
-
- Alavi A, Moallemi N, Zolfaghari G, Amjadi N. A comparison between tocolytic effect of nifedipine and magnesium sulfate in preterm labor pain. International Journal of Gynecology and Obstetrics 2015;131 Suppl 5:E477.
Alavi 2015b {published data only}
-
- Alavi A, Moallemi N, Zolfaghari G, Amjadi N. Effect of maintenance therapy with isoxsuprine in prevention of preterm labor. International Journal of Gynaecology and Obstetrics 2015;131 Suppl 5:E477.
Al Omari 2006 {published data only}
-
- Al-Omari WR, Al-Tikriti E, Al-Shamma H. Atosiban and nifedipine in acute tocolysis, comparative study [abstract]. In: XVIIIth European Congress of Obstetrics and Gynaecology; 2004 May 12-15; Athens, Greece. 2004:103.
Anonymous 2004 {published data only}
-
- Anonymous. TREASURE (Tractocile efficacy assessment survey in Europe) Trial to commence in July. Ferring pharmaceuticals (http://www.ferring.com/) (accessed 24 May 2004) 2004.
Arda 2008 {published data only}
-
- Arda S, Sayin NC, Sut N, Varol FG. The effect of tocolytic agents on maternal and fetal doppler blood flow patterns in women with preterm labor. Journal of Maternal-fetal & Neonatal Medicine 2008;21 Suppl 1:22.
Arikan 1997 {published data only}
-
- Arikan G, Panzitt T, Gucer F, Boritsch J, Trojovski A, Haeusley MC. Oral magnesium supplementation and the prevention of preterm labor. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S45.
Barden 1990 {published data only}
-
- Barden TP [personal communication]. Randomised trial of ritodrine vs placebo in threatened preterm delivery. Letter to: M Keirse (University of Leiden, Netherlands) 30 March 1990.
Bedoya 1972 {published data only}
-
- Bedoya JM. Use of orciprenaline in the treatment of threatened premature labour. In: International Symposium on the Treatment of Fetal Risks; 1972; Baden, Austria. 1972:27-9.
Bivins 1993 {published data only}
-
- Bivins HA, Newman RB, Fyfe DA, Campbell BA, Stramm SL. Randomized comparative trial of indomethacin and terbutaline for the long term treatment of preterm labor. American Journal of Obstetrics and Gynecology 1993;168(1 Pt 2):375. - PubMed
Briscoe 1966 {published data only}
-
- Briscoe CC. Failure of oral isoxsuprine to prevent prematurity. American Journal of Obstetrics and Gynecology 1966;95:885-6. - PubMed
Brown 1981 {published data only}
-
- Brown SM, Tejani NA. Terbutaline sulfate in the prevention of recurrence of premature labor. Obstetrics and Gynecology 1981;57(1):22-5. - PubMed
Bulgay Moerschel 2008 {published data only}
-
- Bulgay-Moerschel M, Schneider U, Schleussner E. Tocolysis with nitroglycerin patches vs. fenoterol i.v. - results of a randomized multicenter study. Journal of Maternal-fetal & Neonatal Medicine 2008;21 Suppl 1:115.
Caballero 1979 {published data only}
-
- Caballero A, Tejerina A, Dominguez A, Nava JM, Caballero A Jr. Indomethacine alone or associated to ritodrine in the prevention of premature labour [abstract]. In: 9th World Congress of Gynecology and Obstetrics; 1979 Oct 26-31; Tokyo, Japan. 1979:300.
Cabero 1988 {published data only}
-
- Cabero L, del-Solar JM, Parra J, Salamero F, Esteban-Altirriba J. Ritodrine retard. A new approach to treatment of threatening premature labour. In: 12th World Congress of Gynecology and Obstetrics; 1988 Oct 23-28; Rio de Janeiro, Brazil. 1988:225.
Calder 1985 {published data only}
-
- Calder AA, Patel NB. Are betamimetics worthwhile in preterm labour? In: Beard RW, Sharp F , editors(s). Preterm Labour and Its Consequences. 13th Study Group of the RCOG. London: RCOG, 1985:209-218.
Caritis 1982 {published data only}
Carr 1999 {published data only}
Castillo 1988 {published data only}
-
- Castillo JM, Alonso J, Hernandez-Garcia JM, Sancho B, Martinez V. Study of biochemical and biophysical modifications produced on pregnant women treated in threatened of premature labor with ritodrine and indometacine. In: 12th World Congress of Gynecology and Obstetrics; 1988 Oct 23-28; Rio de Janeiro, Brazil. 1988:20.
Castren 1975 {published data only}
Cavalle‐Garrido 1997 {published data only}
-
- Cavalle-Garrido T, Panter K, Smallhorn JF, Seaward D, Farine D. A RCT of indomethacin for preterm labor: effects on fetal heart and ductus arteriosus. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S46.
Chau 1992 {published data only}
-
- Chau AC, Gabert HA, Miller JM. A prospective comparison of terbutaline and magnesium for tocolysis. Obstetrics and Gynecology 1992;80(5):847-51. - PubMed
Chawanpaiboon 2009 {published data only}
-
- Chawanpaiboon S, Sutantawibul A, Pimol K, Sirisomboon R, Worapitaksanond S. Preliminary study: comparison of the efficacy of progesterone and nifedipine in inhibiting threatened preterm labour in Siriraj Hospital. Thai Journal of Obstetrics and Gynaecology 2009;17:23-9.
Chhabra 1998 {published data only}
-
- Chhabra S, Patil N. Double blind study of efficacy of isoxsuprine and ritodrine in arrest of preterm labour. Prenatal and Neonatal Medicine 1998;3 Suppl 1:202.
Cifuentes 1994 {published data only}
-
- Cifuentes R, Leon J, De Trochez LM. Comparative study between nifedipine-terbutaline in preterm labor. Revista Colombiana de Obstetricia y Ginecologia 1994;45(2):117-21.
Clavin 1996 {published data only}
-
- Clavin DK, Bayhi DA, Nolan TE, Rigby FB, Cork RC, Miller JM. Comparison of intravenous magnesium sulfate and nitroglycerin for preterm labor: preliminary data [abstract]. American Journal of Obstetrics and Gynecology 1996;174(1 Pt 2):307.
Csapo 1977 {published data only}
-
- Csapo AI, Herczeg J. Arrest of premature labor by isoxsuprine. American Journal of Obstetrics and Gynecology 1977;129:482-8. - PubMed
Danti 2014 {published data only}
Das 1969 {published data only}
-
- Das RK. Isoxsuprine in premature labour. Journal of Obstetrics and Gynaecology of India 1969;19:566-70.
Decavalas 1994 {published data only}
-
- Decavalas G, Papadopoulos V, Tsapanos V, Tzingounis V. Tocolysis in patients with preterm premature rupture of membranes has any effect on pregnancy outcome? International Journal of Gynaecology and Obstetrics 1994;46:26.
Dubay 1992 {published data only}
-
- Dubay P, Singhal D, Bhagoliwal A, Mishra RS. Assessment of new borns of mothers treated with nifedipine and isoxsuprine. Journal of Obstetrics and Gynaecology of India 1992;42(6):778-80.
Dunstan Boone 1990 {published data only}
-
- Dunstan-Boone G, Bond A, Thornton YS. A comparison of verapamil vs ritodrine for the treatment of preterm labor. In: 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23-27; Houston, Texas, USA. 1990:83.
EUCTR2013‐002561‐19‐AT {published data only}
-
- EUCTR2013-002561-19-AT. Does a long term tokolysis with atosiban provide any benefit for the pregnancy outcome, compared to the standard short term tokolysis? http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013-002561-19-AT (date received 2014).
Freeman 2008 {published data only}
-
- Freeman B, Elliott J. Tocolytic therapy in preterm rupture of membranes. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S84.
Fuchs 1976 {published data only}
-
- Fuchs F. Prevention of prematurity. American Journal of Obstetrics and Gynecology 1976;126:809-20. - PubMed
Goodwin 2003 {published data only}
-
- Goodwin TM. Long-term safety with oxytocin antagonists. In: 4th World Congress on Controversies in Obstetrics, Gynecology and Infertility; 2003 April 24-27; Berlin, Germany. 2003:291.
Goyal 2020 {published data only}
-
- Goyal N, Agrawal M. Comparative study of effectiveness of transdermal nitroglycerine patch and oral nifedipine in management of preterm labor. European Journal of Molecular and Clinical Medicine 2020;7(7):2091-8.
Groom 2000 {published data only}
-
- Groom KM, Bennett PR, Shennan AH. Randomised, double-blind, placebo controlled pilot study assessing nitroglycerin as a tocolytic [letter]. BJOG: an international journal of obstetrics and gynaecology 2000;107(9):1182-3. - PubMed
Groom 2005 {published data only}
-
- Groom KM, Shennan AH, Jones BA, Seed P, Bennett PR. TOCOX--a randomised, double-blind, placebo-controlled trial of rofecoxib (a COX-2-specific prostaglandin inhibitor) for the prevention of preterm delivery in women at high risk. BJOG 2005;112(6):725-30. [DOI: 10.1111/j.1471-0528.2005.00539.x] - DOI - PubMed
Guinn 1998 {published data only}
Gummerus 1985 {published data only}
-
- Gummerus M, Halonen O. The value of bed rest and beta-sympathomimetic treatment in multiple pregnancies. Duodecim; Laaketieteellinen Aikakauskirja 1985;101(20):1966-71. - PubMed
Gummerus 1987 {published data only}
Hallak 1992 {published data only}
-
- Hallak M, Moise KJ, Lira N, Dorman K, Smith EO, Cotton DB. The effect of tocolytic agents (indomethacin and terbutaline) on fetal breathing (FBM) and body movements (FM): a prospective, randomized, double blind, placebo-controlled clinical trial. American Journal of Obstetrics and Gynecology 1992;166(1 Pt 2):375. - PubMed
Hallak 1993 {published data only}
-
- Hallak M, Moise KJ, O'Brian Smith E, Cotton DB. The effects of indomethacin and terbutaline on human fetal umbilical artery velocimetry: a randomized, double-blind study. American Journal of Obstetrics and Gynecology 1993;168(1 Pt 2):348. - PubMed
Hobel 1990 {unpublished data only}
-
- Hobel CJ [personal communication]. Randomised trial of ritodrine vs placebo in threatened preterm delivery. Letter to: M Keirse (University of Leiden, Netherlands) 30 March 1990.
Hogberg 1998 {published data only}
-
- Hogberg U [personal communication]. Nitroglycerin and terbutalin versus placebo and terbutalin - a randomized controlled study for preterm labour. Letter to: S Henderson (Cochrane Pregnancy and Childbirth Group, Liverpool, UK) 21 January 1998.
Holleboom 1996 {published data only}
Horton 2012 {published data only}
Horton 2015 {published data only}
How 1994 {published data only}
-
- How H, Allen S, Vogel B, Gall S, Spinnato J. Oral terbutaline in the outpatient management of preterm labor. American Journal of Obstetrics and Gynecology 1994;170:390. - PubMed
How 1995 {published data only}
Husslein 2007 {published data only}
Illia 1993 {published data only}
-
- Illia R, De Diego I, Solana C. Threatened preterm labour. Evaluation of perinatals results in patients treated with betamimetics only and associated with indometacin. Toko-Ginecologia Practica 1993;52:383-7.
IRCT20120215009014N {published data only}
-
- IRCT20120215009014N. Effect of nifedipine with and without sildenafil citrate on management of preterm labor in pregnant women. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20120215009014N382 (first received 2021).
IRCT201204232967N {published data only}
-
- IRCT201204232967N. Effect of Celebrex in prevention of preterm labor. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201204232967N3 (first received 2012).
IRCT201301281760N {published data only}
-
- IRCT201301281760N. Comparison of nifedipin versus magnesium sulfate in treatment of preterm labor. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201301281760N20 (first received 2013).
IRCT2013062613777N1 {published data only}
-
- IRCT2013062613777N1. Comparison of efficacy of indomethacin and magnesium sulphate in prevention of preterm labour. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2013062613777N1 (first received 2013).
Jain 2006 {published data only}
-
- Jain N, Gahlot D. A comparative study of isoxuprine versus ritodrine hydrochloride in the management of preterm labour [abstract]. In: 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6-9; Cochin, Kerala State, India. 2006:148.
Jones 1995 {published data only}
-
- Jones M, Carlan S, Schorr S, McNeill T, Rawji H, Clark K, et al. Oral sulindac to prevent recurrence of preterm labor. American Journal of Obstetrics and Gynecology 1995;172:416. - PubMed
Junejo 2008 {published data only}
-
- Junejo N, Mumtaz F, Unar BA. Comparison of salbutamol and nifedipine as a tocolytic agent in the treatment of preterm labor. Journal of Liaquat University of Medical and Health Sciences 2008;7(2):115-9.
Jung 2020 {published data only}
-
- Jung YM, Lee SM, Kim SM, Kim BJ, Han S, Park JW, et al. 872: a comparison of ritodrine and magnesium sulfate for preterm labor: a randomized clinical trial. American Journal of Obstetrics and Gynecology 2020;222(1):S545. [DOI: 10.1016/j.ajog.2019.11.885] - DOI
-
- NCT02538718. Efficacy and safety of MgSO4 as tocolytics compared to ritodrine in preterm labor. https://clinicaltrials.gov/show/NCT02538718 (first received 2015).
Kashanian 2008 {published data only}
-
- Kashanian M, Soltanzadeh M, Sheikh Ansari N. Atosiban and nifedipin for the treatment of preterm labor. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):69. - PubMed
Kashanian 2015 {published data only}
-
- Kashanian M, Zamen Z, Sheikhansari N. Comparison between nitroglycerin dermal patch and nifedipine for treatment of preterm labor, a randomized clinical trial. International Journal of Gynecology and Obstetrics 2015;131 Suppl 5:E442. - PubMed
Katz 1983 {published data only}
-
- Katz Z, Lancet M, Yemini M, Mogilner BM, Feigl A, Ben-Hur H. Treatment of premature labor contractions with combined ritodrine and indomethacine. International journal of Gynecology and Obstetrics 1983;21:337-42. - PubMed
Kawagoe 2011 {published data only}
Khuteta 1988 {published data only}
-
- Khuteta RP, Garg S, Bhargava A. Mefanimic acid in prevention of premature labour. In: 12th FIGO World Congress of Gynecology and Obstetrics; 1988 October 23-28; Brazil. 1988:222.
Kim 1983 {published data only}
-
- Kim MH, Sch BH, Lee JH. The clinical study of ritodrine hydrochloride (Yutopar). Effect on preterm labour. Korean Journal of Obstetrics and Gynecology 1983;26:23-32.
Kosasa 1985 {published data only}
Kullander 1985 {published data only}
Kurki 1991a {published data only}
Lauersen 1977 {published data only}
Leake 1980a {published data only}
-
- Leake RD, Hobel CJ, Oh W, Thibeault DW, Okada DM, Williams PR. A controlled, prospective study of the effects of ritodrine hydrochloride for premature labor. Clinical Research 1980;28(1):90A.
Leake 1980b {published data only}
-
- Leake RD, Hobel CJ, Oh W, Thibeault DW, Okada DM, Williams PR. A controlled, prospective study of the effect of ritodrine hydrochloride (R) for premature labor. Pediatric Research 1980;14:603.
Lenzen 2012 {published data only}
-
- Lenzen V, Bartz C, Rath WH. Atosiban versus fenoterol treatment of pre-term labour: randomised, prospective, multicentre study. Archives of Gynecology and Obstetrics 2012;286 Suppl 1:S197-S198.
Levy 1985 {published data only}
-
- Levy DL, Warsof SL. Oral ritodrine and preterm premature rupture of membranes. Obstetrics and Gynecology 1985;66(5):621-3. - PubMed
Lewis 1996 {published data only}
-
- Lewis R, Mercer B, Salama M, Walsh M, Sibai B. Oral terbutaline after parenteral tocolysis: a randomized, double-blind, placebo-controlled trial. American Journal of Obstetrics and Gynecology 1996;174(1 Pt 2):315. - PubMed
Lorzadeh 2007 {published data only}
-
- Lorzadeh N, Kazemirad S, Lorzadrh M, Dehnori A. A comparison of human chorionic gonadotropin with magnesium sulphate in inhibition of preterm labor. Journal of Medical Sciences (Taipei, Taiwan) 2007;7(4):640-4.
Lumme 1991 {published data only}
-
- Lumme R, Kurki T, Pyorala T, Ylikorkala O. Indomethacin is more effective than nylidrine in arresting preterm labor. In: 2nd European Congress on Prostaglandins in Reproduction; 1991 April 30-May 3; the Hague, Netherlands. 1991:202.
Lyell 2007b {published data only}
-
- Lyell D, Pullen K, Mannan J, Chitkara U, Druzin ML, Caughey A, et al. Maintenance nifedipine vs. placebo: a prospective, double blind trial. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S6, Abstract no: 10.
Lyell 2008 {published data only}
-
- NCT00185952. Nifedipine vs placebo for maintenance tocolysis of preterm labor. https://clinicaltrials.gov/show/NCT00185952 (first received 2005).
Lyell 2009 {published data only}
-
- Lyell D, Penn A, Caughey A, Kogut E, McClellan L, Adams B, et al. Neonatal outcomes following antenatal magnesium sulfate exposure: follow up from a magnesium vs. nifedipine tocolysis RCT. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S180-S181.
Ma 1992 {published data only}
-
- Ma L. Magnesium sulfate in prevention of preterm labor. Chung-hua-i-hsueh-tsa-chih-taipei 1992;72(3):158-161, 191. - PubMed
Maitra 2007 {published data only}
-
- Maitra N, Christian V, Verma RN, Desai VA. Maternal and fetal cardiovascular side effects of nifedipine and ritodrine used as tocolytics. Journal of Obstetrics and Gynaecology of India 2007;57(2):131-4.
Malik 2007 {published data only}
-
- Malik KK. Comparison of nifedipine with salbutamol as tocolytic agents in preterm labour. Biomedica 2007;23:111-5.
Mariona 1980 {published data only}
-
- Mariona A [personal communication]. Randomised trial of ritodrine vs placebo in threatened preterm delivery. Randomised trial of ritodrine vs placebo in threatened preterm delivery 1980.
Martin 1990 {published data only}
-
- Martin RW, McColgin SW, Perry KG, McCaul JF, Hess LW, Martin JN, et al. Oral magnesium and the prevention of preterm labor in a high-risk group of patients. In: 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23-27; Houston, Texas, USA. 1990:181.
Martin 1992 {published data only}
Martinez 1994 {published data only}
-
- Martinez S, Manau MD, Vives A, Carmona F, Deulofeu P, Cararach V. A prospective and randomized study about the use of calcium blockers vs betamimetics in preterm labour. In: 14th European Congress of Perinatal Medicine;1994 June 5-8; Helsinki, Finland. 1994:Abstract no: 414.
Mathew 1997 {published data only}
-
- Ashok MS. A comparative study of tocolytic effect of nifedipine & isox suprine hydrochloride. Acta Obstetricia et Gynecologica Scandinavica 1997;76(167):90.
Mathews 1967 {published data only}
Matijevic 2006 {published data only}
-
- NCT00290173. Ritodrine in oral maintenance of tocolysis after active preterm labor. https://clinicaltrials.gov/show/NCT00290173 (first received 2006). - PMC - PubMed
Merkatz 1980 {published data only}
-
- Merkatz IR, Peter JB, Barden TP. Ritodrine hydrochloride: a betamimetic agent for use in preterm labor. II. Evidence of efficacy. Obstetrics and Gynecology 1980;56(1):7-12. - PubMed
Mittendorf 1997 {published data only}
Mittendorf 2002 {published data only}
-
- Mittendorf R, Dambrosia J, Khoshnood B, Lee KS, Pryde P, Yousefzadeh D. Magnesium sulfate is no more efficacious than other tocolytic agents [abstract]. American Journal of Obstetrics and Gynecology 2001;184(1):S188.
Morales 1993 {published data only}
-
- Morales W, Madhav H. Efficacy and safety of indomethacin vs magnesium sulfate in the management of preterm labor: a randomized study. American Journal of Obstetrics and Gynecology 1991;164:280. - PubMed
Motazedian 2010 {published data only}
Moutquin 1997 {published data only}
-
- Moutquin JM, Rabinovici J. Comparison of atosiban versus ritodrine in the treatment of pre-term labour. Acta Obstetricia et Gynecologica Scandinavica 1997;76(167):33.
Na Nan 2018 {published data only}
NCT00116623 {published data only}
-
- NCT00116623. Magnesium sulfate versus indomethacin for preterm labor. https://clinicaltrials.gov/show/NCT00116623 (first received 2005).
NCT00463736 {published data only}
-
- NCT00463736. Magnesium sulfate versus placebo for tocolysis in PPROM. https://clinicaltrials.gov/ct2/show/NCT00463736 (first received 2011).
NCT00525486 {published data only}
-
- NCT00525486. Extended release nifedipine treatment as maintenance tocolysis to prevent preterm delivery. https://clinicaltrials.gov/show/NCT00525486 (first received 2007).
NCT00620724 {published data only}
-
- NCT00620724. Tocolytic therapy in conservative management of symptomatic placenta previa. https://clinicaltrials.gov/show/NCT00620724 (first received 2008). - PubMed
NCT00641784 {published data only}
-
- NCT00641784. Tocolytics trial: intravenous (IV) magnesium versus oral nifedpine in fetal fibronectin (FFN) postive population. https://clinicaltrials.gov/show/NCT00641784 (first received 2008).
NCT01314859 {published data only}
-
- NCT01314859. Nifedipine treatment in preterm labor. https://clinicaltrials.gov/show/NCT01314859 (first received 2011).
NCT01360034 {published data only}
-
- NCT01360034. Nifedipine versus indomethacin in the treatment of preterm labour. https://clinicaltrials.gov/show/NCT01360034 (first received 2011).
NCT01577121 {published data only}
-
- NCT01577121. Evaluation of the use of indomethacin as co-treatment in women with preterm labor and high risk of intraamniotic inflammation. https://clinicaltrials.gov/show/NCT01577121 (first received 2012).
NCT01796522 {published data only}
-
- NCT01796522. Utility of tocolytic therapy for maintenance tocolysis in the management of threatened preterm delivery. https://clinicaltrials.gov/show/NCT01796522 (first received 2013).
NCT01985594 {published data only}
-
- NCT01985594. Utrogestan versus nifedipine as tocolysis for preterm labor: a randomised controlled trial. https://clinicaltrials.gov/show/NCT01985594 (first received 2013).
NCT02438371 {published data only}
-
- NCT02438371. Nifedipine or nifedipine plus indomethacin for treatment of acute preterm labor. https://clinicaltrials.gov/show/NCT02438371 (first received 2015).
NCT02583633 {published data only}
-
- NCT02583633. Transdermal nitroglycerin and nifedipine in preterm labor. https://clinicaltrials.gov/show/NCT02583633 (first received 2015).
NCT03040752 {published data only}
-
- NCT03040752. Comparative study between nifedipine and ritodrine as maintenance tocolytic therapy in preterm labor. https://clinicaltrials.gov/show/NCT03040752 (first received 2017).
Nelson 1985 {published data only}
-
- Nelson LH, Meis PJ, Hatjis CG, Ernest JM, Dillard R, Schey HM. Premature rupture of membranes: a prospective, randomized evaluation of steroids, latent phase, and expectant management. Obstetrics and Gynecology 1985;66(1):55-8. - PubMed
Neri 2008 {published data only}
-
- Neri I, Monari F, Valensise H, Facchinetti F, Bellafronte M, Volpe A. Computerized evaluation of fetal heart rate during tocolytic treatment: comparison between atosiban and ritodrine. Journal of Maternal-fetal & Neonatal Medicine 2008;21 Suppl 1:22. - PubMed
Nevils 1994 {published data only}
-
- Nevils B, Curet L, Izquierdo L, Chatterjee M, Gilson G, Maciulla J, et al. Indomethacin as a 'rescue' tocolytic in preterm labor. American Journal of Obstetrics and Gynecology 1994;170:378.
Newton 1991 {published data only}
OConnor 1979 {published data only}
Panter 1999 {published data only}
-
- Panter K, Hannah M, Farine D, Amankwah K, Jeffries A, Ohlsson A. The effect of indomethacin tocolysis of preterm labor on perinatal outcome: a RCT. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S46. - PubMed
-
- Panter KR, Hannah ME, Amankwah KS, Ohlsson A, Jefferies A, Farine D. The effect of indomethacin tocolysis in preterm labour on perinatal outcome: a randomised placebo-controlled trial. British Journal of Obstetrics and Gynaecology 1999;106(5):467-73. [DOI: 10.1111/j.1471-0528.1999.tb08300.x] - DOI - PubMed
Papadopoulos 1997 {published data only}
-
- Papadopoulos V, Decavalas G, Tzingounis V. Nifedipine versus ritodrine in the treatment of preterm labor. Acta Obstetricia et Gynecologica Scandinavica. Supplement 1997;76(167:1):88.
Papatsonis 1997a {published data only}
-
- Papatsonis DN, Kok JH, Samson JF, Lange FM, Ader HJ, Dekker GA. Neonatal morbidity after randomized trial comparing nifedipine with ritodrine in the management of preterm labor. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S117.
Parilla 1993 {published data only}
-
- Parilla B, Dooley S, Socol M. The efficacy of oral terbutaline following parental tocolysis for preterm labor [abstract]. American Journal of Obstetrics and Gynecology 1993;168:376. - PubMed
Park 1982 {published data only}
-
- Park IS, Kim YC, Park HM, Cha IS. Effect of ritodrine hydrochloride (Yutopar) on premature labor. Korean Journal of Obstetrics and Gynecology 1982;25:935-44.
Parry 2014 {published data only}
-
- Parry E, Roos C, Stone P, Hayward L, Mol BW, McCowan L. The NIFTY study: a multi-centre randomised double blind placebo controlled trial of nifedipine maintenance tocolysis in fetal fibronectin positive women in threatened preterm labour. American Journal of Obstetrics and Gynecology 2012;206 Suppl 1:S216. [DOI: ] - PubMed
-
- Parry E, Roos C, Stone P, Hayward L, Mol BW, McCowan L. The NIFTY study: a multicentre randomised double-blind placebo-controlled trial of nifedipine maintenance tocolysis in fetal fibronectin-positive women in threatened preterm labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 2014;54(3):231-6. [DOI: 10.1111/ajo.12179] - DOI - PubMed
Parsons 1988 {published data only}
-
- Parsons MT, Sobel D, Cummiskey K, Constantine L, Roitman J. Steroid, antibiotic and tocolytic vs no steroid, antibiotic and tocolytic management in patients with preterm PROM at 25-32 weeks. In: 8th Annual Meeting of the Society of Perinatal Obstetricians; 1988 Feb 3-6; Las Vegas, Nevada, USA. 1988:44.
Pasargiklian 1983 {published data only}
-
- Pasargiklian R, Monti G, Bertulessi C. Clenbuterol in the treatment of premature labor. Minerva Ginecologica 1983;35(6):423-9. - PubMed
Poppiti 2009 {published data only}
-
- Poppiti R, Nazzaro G, De Placido G, Palmieri T, Locci M. Prevention of preterm delivery in twin pregnancies with atosiban. Journal of Maternal-fetal & Neonatal Medicine 2009;22 Suppl 1:61.
Purwaka 2004 {published data only}
-
- Purwaka BT, Abadi A, Prasetyadi FOH. Comparison of efficacy between oral nimesulide and parenteral-oral isoxsuprine to delay threatened preterm labour [abstract]. Journal of Obstetrics and Gynaecology Research 2004;30(3):264.
Rashid 2018 {published data only}
-
- Rashid M. Prevention of premature labour with MgSO4-a better option. International Journal of Gynecology and Obstetrics 2018;143 Suppl 3:201. [DOI: 10.1002/ijgo.12582] - DOI
Rath 2006 {published data only}
-
- Rath S. Use of glyceryl trinitrate for preterm labour in a maternity hospital in United Arab Emirates. In: 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6-9; Cochin, Kerala State, India. 2006:49.
Rezk 2015 {published data only}
Ricci 1990 {published data only}
-
- Ricci JM, Hariharan S, Helfgott A, Reed K, O'Sullivan MJ. Oral tocolysis with magnesium chloride: a randomized controlled prospective clinical trial. In: 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23-27; Houston, Texas, USA. 1990:156. - PubMed
Ridgway 1990 {published data only}
-
- Ridgway LE, Muise K, Patterson RM, Wright JW, Newton E, Gibbs RS. A prospective randomized comparison of oral terbutaline and magnesium oxide for the maintenance of tocolysis. In: 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 January 23-27; Houston, Texas, USA. 1990:170. - PubMed
Rios Anez 2001 {published data only}
-
- Rios-Anez R, Santos-Luque M, Noguera ME. Use of an antagonist of the prostaglandins associated to a b-sympathomimetic agent in the treatment of pre-term labour. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 1):165.
Roos 2013 {published data only}
-
- NTR1336. Assessment of perinatal outcome with sustained tocolysis in early labour. http://www.who.int/trialsearch/Trial2.aspx?TrialID=NTR1336 (first received 2008). - PMC - PubMed
-
- Roos C. The effectiveness of maintenance tocolysis with nifedipine in threatened preterm labor, a randomized placebo-controlled trial. Journal of Maternal-fetal & Neonatal Medicine 2012;25:46. [DOI: ]
Roy 2006 {published data only}
-
- Roy V, Prasad GS, Latha K. Tocolysis with ritodrine: a comparative study in preterm labour. Pakistan Journal of Medical Sciences 2006;22(1):64-9.
Rust 1996 {published data only}
Ryden 1977 {published data only}
-
- Ryden G. The effect of salbutamol and terbutaline in the management of premature labour. Acta Obstetricia et Gynecologica Scandinavica 1977;56:293-6. - PubMed
Sanchez Ramos 1997 {published data only}
-
- Sanchez-Ramos L, Valenzuela G, Romero R, Silver H, Koltun W, Millar L, et al. A double-blind placebo-controlled trial of oxytocin receptor antagonist (antocin) maintenance therapy in patients with preterm labor. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S30.
Sauve 1991 {published data only}
-
- Sauve RS. Outcomes of ritodrine exposed infants. Pediatric Research 1991;29 Suppl:264A.
Sayin 2004 {published data only}
Sciscione 1993 {published data only}
-
- Sciscione A, Gorman R, Schlossman P, Colmorgen G. A randomized prospective study of intravenous magnesium sulfate, ritrodine, and subcutaneous terbutaline as treatments for preterm labor. American Journal of Obstetrics and Gynecology 1993;168:376 [SPO Abstract 281].
Sharma 2000 {published data only}
-
- Sharma A. A randomized comparison of nifedipine and ritodrine for suppression of preterm labour. In: XVI FIGO World Congress of Obstetrics & Gynecology (Book 2); 2000 Sept 3-8; Washington Dc, USA. 2000:156.
Shrivastava 2008 {published data only}
-
- Shrivastava V, Ehsanipoor R, Lee RM, Chan K, Gaylean A, Garite T, et al. Randomized double-blinded trial of indomethacin tocolysis versus expectant management in patients with premature rupture of membranes at 24-32 weeks of gestation. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S59.
Silver 1997 {published data only}
-
- Silver H, Valenzuela G, Sanchez-Ramos L, Romero R, Sibai B, Goodwin T, et al. Maternal side effects and safety of the oxytocin receptor antagonist antocin. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S45.
Singh 2011 {published data only}
Sirohiwal 2001 {published data only}
-
- Sirohiwal D, Sachan A, Bano A, Gulati N. Tocolysis with ritodrine: a comparative study in preterm labour. Journal of Obstetrics and Gynaecology of India 2001;51(3):66-7.
Smit 1983 {published data only}
-
- Smit DA. Efficacy of Orally Administered Ritodrine after Initial Intravenous Therapy [thesis]. Limburg (The Netherlands): University of Limburg, 1983.
Smith 1993 {published data only}
Snyder 1989 {published data only}
-
- Snyder S [personal communication]. Nifedipine and magnesium sulfate as tocolytics. Trial to compare the efficacy of nifedipine and magnesium sulfate as tocolytics 1989.
Sofat 1994 {published data only}
-
- Sofat R, Gill BK, Goyal A. Comparison of nifedipine and isoxsuprine in the arrest of preterm labour. International Journal of Gynecology and Obstetrics 1994;46 Suppl:59.
Spatling 1989 {published data only}
-
- Spatling L, Fallenstein F, Schneider H, Dancis J. Bolus tocolysis: treatment of preterm labor with pulsatile administration of a beta-adrenergic agonist. American Journal of Obstetrics and Gynecology 1989;160:713-7. - PubMed
Spearing 1979 {published data only}
-
- Spearing G. Alcohol, indomethacin and salbutamol. A comparative trial of their use in preterm labour. Obstetrics and Gynecology 1979;53:171-4. - PubMed
Stika 2002 {published data only}
Thornton 2017 {published data only}
Uma 2012 {published data only}
-
- Uma M, Ixora KA, Nor Azlin MI, Mahady ZA. Maintenance nifedipine for tocolysis in preterm labour: a prospective randomised controlled trial. BJOG 2012;119 Suppl:35.
Valenzuela 2000 {published data only}
-
- Valenzuela GJ, Sanchez-Ramos L, Romero R, Silver HM, Koltun WD, Millar L, et al. Maintenance treatment of preterm labor with the oxytocin antagonist atosiban. The Atosiban PTL-098 Study Group. American Journal of Obstetrics and Gynecology 2000;182(5):1184-90. [DOI: 10.1067/mob.2000.105816] - DOI - PubMed
Verspyck 2017 {published data only}
-
- Verspyck E, Vienne C, Muszynski C, Bubenheim M, Chanavaz-Lacheray I, Dreyfus M, et al. Maintenance nifedipine therapy for preterm symptomatic placenta previa: a randomized, multicenter, double-blind, placebo-controlled trial. PloS One 2017;12(3):e0173717. [DOI: 10.1371/journal.pone.0173717] - DOI - PMC - PubMed
Verspyck 2018 {published data only}
-
- Verspyck E, De Vienne C, Muszynski C, Bubenheim M, Chanavaz-Lacheray I, Dreyfus M, et al. Conservative management of preterm bleeding placenta previa by nifedipine therapy. International Journal of Gynaecology and Obstetrics 2018;143 Suppl 3:119. [DOI: 10.1002/ijgo.12583] - DOI
Vis 2009 {published data only}
-
- Vis JY, Wilms FF, Oudijk MA, Porath MM, Scheepers HC, Bloemenkamp KW, et al. Cost-effectiveness of fibronectin testing in a triage in women with threatened preterm labor: alleviation of pregnancy outcome by suspending tocolysis in early labor (APOSTEL-I trial). BMC Pregnancy and Childbirth 2009;9:38. [DOI: 10.1186/1471-2393-9-38] - DOI - PMC - PubMed
Von Oeyen 1990 {published data only}
-
- Von Oeyen P, Braden G, Smith M, Garb J, Liucci L. A randomized study comparing ritodrine and terbutaline for the treatment of preterm labor. In: 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23-27; Houston, Texas, USA. 1990:186.
Wani 1999 {published data only}
-
- Wani M, John S, Graham I. A comparison of glyceryl trinitrate and ritodrine for the treatment of threatened preterm labour. In: Women's Health - into the New Millennium. 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3-6; Cape Town South Africa. 1999:62.
Weiner 1988 {published data only}
Weisbach 1986 {published data only}
-
- Weisbach W, Wagner F, Jager KH. Oral long-term tocolysis of threatened premature labor using clenbuterol (Contraspasmin, Spiropent). A placebo-controlled double-blind method [Orale Langzeittokolyse der drohenden Fruhgeburt mit Clebuterol (Contraspasmin (R), Spiropent (R)). Eine plazebokontrolliert Doppelblindstudie]. Zentralblatt fur Gynakologie 1986;108(7):419-23. - PubMed
Wenstrom 1997 {published data only}
-
- Wenstrom K, Weiner C, Merrill D, Niebyl J. A placebo-controlled trial of the terbutaline (T) pump for prevention of preterm delivery. American Journal of Obstetrics and Gynecology 1995;172:416. - PubMed
Wesselius De Casparis 1971 {published data only}
Woodland 1990 {published data only}
-
- Woodland MB, Smith C, Byers J, Bolognese R, Weiner S. Clinical comparison of oral nifedipine and subcutaneous terbutaline use for initial tocolysis. In: 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23-27; Houston, Texas, USA. 1990:523.
Yi 1991 {published data only}
-
- Yi CS, Kim DK. A comparison of tocolytic effects of ritodrine hydrochloride and nifedipine in the treatment of preterm labour. Journal of Catholic Medical College 1991;41(1):231-8.
Zarcone 1994 {published data only}
-
- Zarcone R, Cardone G, Bellini P. Role of magnesium in pregnancy. Panminerva Medica 1994;36(4):168-70. - PubMed
Zygmunt 2003 {published data only}
References to studies awaiting assessment
Akhtar 2018 {published data only}
-
- Akhtar R, Awais M, Furqan A. Comparing the efficacy of nifedipine and nitroglycerine as tocolytic agent in preterm labor patients. Rawal Medical Journal 2018;43(2):280-4.
Ali 2013 {published data only}
-
- Ali M, Hussain MA, Qaisrani HG, Chohan MA. To compare the efficacy of beta agonist ritodrine and calcium channel blocker nifedipine in the management of preterm labour. Pakistan Journal of Medical and Health Sciences 2013;7(4):1167-9.
Al Jawady 2020 {published data only}
-
- Al Jawady SA, Al Sanjary AA. Comparative study between atosiban and salbutamol in treatment of preterm labor. Pakistan Journal of Medical and Health Sciences 2020;14(2):1068-71.
Aziz 2018 {published data only}
-
- Aziz H, Batool S, Hashmi KS. Comparison of efficacy of magnesium sulphate versus nifedipine for tocolysis of preterm labour. Pakistan Journal of Medical and Health Sciences 2018;12(4):1669-72.
Badshah 2019 {published data only}
-
- Badshah MK, Utman N, Ara J, Shahab T, Khattak R. Comparison of nitroglycerine VS nifedipine for preterm labour. Medical Forum Monthly 2019;30(4):25-8.
Bina 2012 {published data only}
-
- Bina I, Parveen T, Khanom A, Shamsunnahar PA. The tocolytic role of nifedipine in preventing preterm labour pain. Mymensingh Medical Journal : MMJ 2012;21(1):139-44. - PubMed
-
- Bina I. The tocolytic role of nifedipine in preventing preterm labour pain: a study in a developing country like Bangladesh. International Journal of Gynecology and Obstetrics 2015;131(S5):E112.
Caliskan 2015 {published data only}
Chawanpaiboon 2011 {published data only}
Chawanpaiboon 2012 {published data only}
-
- Chawanpaiboon S, Kanokpongsakdi S. Comparison of nifedipine and bed rest for inhibiting threatened preterm labour. Gynecology and Obstetrics 2012;2(5):1-4.
Dhawle 2013 {published data only}
-
- Dhawle A, Kalra J, Bagga R, Aggarwal N. Nifedipine versus nitroglycerin for acute tocolysis in preterm labour: a randomised controlled trial. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2013;2(1):61-6.
Eftekhari 2012 {published data only}
-
- Eftekhari E. Intravenous magnesium sulfate compared with oral indomethacin in preterm labor. International Journal of Gynecology and Obstetrics 2012;119:S333-S334. [DOI: ]
-
- Eftekhari N, Pour Rahimi M. Comparison of the efficacy of intravenous magnesium sulfate and oral indomethcin in the management of preterm labor. Journal of Kerman University of Medical Sciences 2012;19(3):287-99.
Esmaeilzadeh 2017 {published data only}
-
- Esmaeilzadeh S, Ramezani M, Pahlevan Z, Taheri S, Zabihi Naeimirad M. Nifedipin versus magnesium sulfate for suppression of preterm labor: a randomized clinical trial. Caspian Journal of Reproductive Medicine 2017;31(1):25-30.
Faisal 2020 {published data only}
-
- Faisal J, Kanwal S, Inayat FC, Jawad Z, Shabana N, Zafar I. Comparison of magnesium sulfate and nifedipine for the management of preterm labour. Pakistan Journal of Medical and Health Sciences 2020;14(2):534-7.
Faraji 2013 {published data only}
-
- Faraji R, Asgharnia M, Dalil Heirati SF, Nemati F. A comparison between magnesium sulfate and nifedipine for preterm labor prevention. Journal of Babol University of Medical Sciences 2013;15(4):88-92.
-
- IRCT2012062310089N. Magnesium sulfate and nifedipine on treatment of preterm labor. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2012062310089N1 (first received 2013).
Ghomian 2015 {published data only}
-
- Ghomian N, Vahedalain SH, Tavassoli F, Pourhoseini SA, Heydari ST. Transdermal nitroglycerin versus oral nifedipine for suppression of preterm labor. Shiraz E-Medical Journal 2015;16(11-12):e31018. [DOI: ]
Hamza 2016 {published data only}
-
- Hamza S, Ahuja K, Asghar U. Comparison of efficacy of transdermal nitroglycerine patch and intravenous ritodrine in preterm labor. Medical Forum Monthly 2016;27(11):41-4.
IRCT2015042621947N1 {published data only}
-
- IRCT2015042621947N1. Effect of celecoxib in order to prevent spontaneous preterm labor. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2015042621947N1 (first received 2016).
Jamil 2020 {published data only}
-
- Jamil M, Abid R, Basharat A, Kharunisa. Transdermal nitro-glycerine versus oral nifedipine for acute tocolysis in preterm labour: a randomised controlled trial. Journal of the Society of Obstetricians and Gynaecologists of Pakistan 2020;10(1):26-9.
-
- Jamil M, Basharat A, Ayub S. Transdermal nitro-glycerine versus oral nifedipine for acute tocolysis in preterm labour: a randomised controlled trial. International Journal of Gynecology and Obstetrics 2015;131 Suppl 5:E492.
Khooshideh 2017 {published data only}
-
- IRCT2016120711020N8. Effect of nifedipin and magnesium sulfate on preterm labor. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2016120711020N8 (first received 2017).
-
- Khooshideh M, Rahmati J, Teimoori B. Nifedipine versus magnesium sulfate for treatment of preterm labor: comparison of efficacy and adverse effects in a randomized controlled trial. Shiraz E-Medical Journal 2017;18(6):e46875. [DOI: 10.5812/semj.46875] - DOI
Kim 2001 {published data only}
-
- Kim JH, Ahn KH, Kim JY, Jeong YJ, Cho SN. A comparison for efficacy and safety of magnesium sulfate (Magrose), ritodrine hydrochloride (Yutopar) and nifedipine (Adalat) in the management of preterm labor. Korean Journal of Obstetrics and Gynecology 2001;44(6):1165-70.
Lee 2004 {published data only}
-
- Lee BK, Park IW. Doppler findings and tocolytic effect of transdermal glyceryl trinitrate and intravenous ritodrine as tocolysis of preterm labor. Korean Journal of Obstetrics and Gynecology 2004;47(12):2447-52.
Lotfalizadeh 2010 {published data only}
-
- Lotfalizadeh M, Teymoori M. Comparison of nifedipine and magnesium sulfate in the treatment of preterm birth. Iranian Journal of Obstetrics, Gynecology and Infertility 2010;13(2):7-12.
Madkour 2013 {published data only}
-
- Madkour W, Abdelhamid AM. Is combination therapy of atosiban and nifedipine more effective in preterm labor than each drug alone? A prospective study. Current Women's Health Reviews 2013;9(4):209-14. [DOI: ]
Mesdaghinia 2012 {published data only}
-
- IRCT138811223329N1. Comparison of delaying in premature labour by indomethacin and Mg sulfate in pregnant women referred to maternity hospital. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT138811223329N1 (first received 2010).
-
- Mesdaghinia E, Mesdaghinia A, Hashemi T, Sooky Z, Mousavi GA. Comparing the effects of indomethacin and magnesium-sulfate in the treatment of preterm labor. Feyz, Journal of Kashan University of Medical Sciences 2012;16(2):95-101.
Mirteimoori 2009 {published data only}
-
- Mirteimoori M, Sakhavar N, Teimoori B. Glyceryl trinitrate versus magnesium sulfate in the suppression of preterm labor. Shiraz E-Medical Journal 2009;10(2):73-8.
Mirzamoradi 2014 {published data only}
-
- Mirzamoradi M, Behnam M, Jahed T, Saleh-Gargari S, Bakhtiyari M. Does magnesium sulfate delay the active phase of labor in women with premature rupture of membranes? A randomized controlled trial. Taiwanese Journal of Obstetrics & Gynecology 2014;53(3):309-12. [DOI: 10.1016/j.tjog.2013.06.014] - DOI - PubMed
-
- Mirzamoradi M, Kimyaiee P, Mansouri A, Bakhtiyari Z, Bakhtiyari M. The effect of magnesium sulfate in delaying delivery in premature rupture of membrane and its fetal complications. Iranian Journal of Obstetrics, Gynecology and Infertility 2014;17(127):1-9.
Nankali 2014 {published data only}
-
- IRCT201108054025N3. Investigation of transdermal nitroglycerine effect on preterm labor. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201108054025N3 (first received 2011).
Nauman 2020 {published data only}
-
- Nauman D, Saif N, Sukhan S, Saghir F, Farooq F, Rashid M. Comparison of efficacy and safety of nifedipine versus beta-sympathomimetics for suppression of preterm labour. Medical Forum Monthly 2020;31(5):57-61.
NCT00486824 {published data only}
-
- NCT00486824. Indomethacin versus nifedipine for preterm labor tocolysis. https://clinicaltrials.gov/show/NCT00486824 (first received 2007).
Nikbakht 2014 {published data only}
-
- IRCT2013090914603N1. Treating preterm labor by nifedipine or magnesium sulfate. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2013090914603N1 (first received 2013).
Ozhan Baykal 2015 {published data only}
PriyadarshiniBai 2013 {published data only}
-
- Priyadarshini Bai G, Ravikumar P, Padma L. A comparative study of safety and efficacy of ritodrine versus nifedipine in the management of preterm labor. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2013;4(3):1388-97.
Saadati 2014 {published data only}
Sachan 2012 {published data only}
-
- Sachan R, Gupta P, Patel ML, Chaudhary S, Agarwal R. Clinical evaluation of transdermal nitroglycerine in preterm labor in tertiary care teaching hospital in North India. International Journal of Scientific and Research Publications 2012;2(3):1-7.
Shafaie 2014 {published data only}
-
- IRCT201201308878N1. Magnesium sulfate in adverse with nifedipine in suppression preterm labor in pregnant with preterm labor. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201201308878N1 (first received 2012).
-
- Shafaie FS, Fartash F, Fardiazar Z, Gojazadeh M. Fetal & neonatal outcomes of the magnesium sulfate and nefidipine in suppression of preterm labor. International Journal of Women's Health and Reproduction Sciences 2014;2(3):155-9. [DOI: ]
-
- Shafayi FS, Fartash F, Qoujazadeh M, Azar ZF. Maternal outcomes caused by receiving magnesium sulfate to suppress preterm labor. Iranian journal of obstetrics, gynecology and infertility 2014;17(112):1-6.
Shirazi 2015 {published data only}
-
- Shirazi A, Ansari NU, Ahmed M. Comparison of efficacy for tocolysis of preterm labour between magnesium sulphate and nifedipine. Pakistan Journal of Medical and Health Sciences 2015;9(4):1177-80.
Song 2002a {published data only}
-
- Song TB, Kim YH, Na JH, KimYS, Kang WD, Oh YS, et al. Oral nicardipine versus intravenous MgSO4 for the treatment of preterm labor. Chonnam Medical Journal 2002;38(4):359-63.
Song 2002b {published data only}
-
- Song TB, Kim YH, Choi J, Kang WD, Oh YS, Kang MS, et al. Oral nicardipine versus intravenous ritodrine for the treatment of preterm labor. Korean Journal of Obstetrics and Gynecology 2002;45(12):2153-57.
Songthamwat 2018 {published data only}
Tabassum 2016 {published data only}
-
- Tabassum S, Shahzadi U, Khalid A. Comparative study of efficacy of magnesium sulfate & nifedipine in suppression of preterm labour. Pakistan Journal of Medical and Health Sciences 2016;10(4):1307-11.
Toghroli 2020 {published data only}
-
- Toghroli H, Saeieh SE, Rezapour-Nasrabad R, Rahimzadeh M, Ataei M, Faraji A. Comparative study of the tocolytic effects of magnesium sulfate and indomethacin on pregnancy duration and neonatal outcomes in preterm labor: a clinical trial. International journal of Pharmaceutical Research 2020;12(3):559-64. [DOI: 10.31838/ijpr/2020.12.03.082] - DOI
Xu 2016 {published data only}
-
- Xu YJ, Ran LM, Zhai SS, Luo XH, Zhang YY, Zhou ZY, et al. Evaluation of the efficacy of atosiban in pregnant women with threatened preterm labor associated with assisted reproductive technology. European Review for Medical and Pharmacological Sciences 2016;20(9):1881-7. - PubMed
Yasmin 2016 {published data only}
-
- Yasmin S, Sabir S, Zahoor F. To compare the effectiveness of nifedipine and glyceryl trinitrate patch in prevention of preterm labour. Journal of Postgraduate Medical Institute 2016;30(1):92-6.
Zangooei 2011 {published data only}
-
- IRCT201105226558N1. The effect of corticosteroid, antibiotic and tocolytic on neonatal outcome. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201105226558N1 (first received 2011).
-
- Zangooei M, Sharifzadeh GR, Karimi A, Gheytas H. The effect of antibiotic, corticosteroid and tocolytic in patient with PPROM on neonatal outcomes. Modern Care Journal 2011;8(1):19-24.
References to ongoing studies
CTRI/2017/11/010518 {published data only}
-
- CTRI/2017/11/010518. Atosiban (6.75 mg) injection to delay preterm birth. http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/11/010518 (first received 2017).
EUCTR2007‐004506‐27‐FR {published data only}
-
- EUCTR2007-004506-27-FR. Interest of tocolysis in the management of premature rupture of membranes between 24 and 34 weeks of amenorrhea - TOCOPREMA [Interet de la tocolyse dans la prise en charge des ruptures prematurees des membranes entre 24 et 34 semaines d’amenorrhee. - TOCOPREMA]. https://www.clinicaltrialsregister.eu/ctr-search/search?query=2007-00450... (first received 2007).
EUCTR2017‐002579‐25‐FI {published data only}
-
- EUCTR2017-002579-25-FI. OBE022 added-on to atosiban in threatened spontaneous preterm labour, proof of concept study. http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2017-002579-25-FI (first received 2017).
-
- NCT03369262. PoC study of OBE022 in threatened preterm labour. https://clinicaltrials.gov/show/NCT03369262 (first received 2017).
EUCTR2018‐004482‐14‐FR {published data only}
-
- EUCTR2018-004482-14-FR. Tocolysis in the management of preterm premature rupture of membranes before 34 weeks of gestation: a double-blinded randomized controlled trial - TOCOPROM. http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2018-004482-14-FR (first received 2019).
-
- NCT03976063. Tocolysis in the management of preterm premature rupture of membranes before 34 weeks of gestation (TOCOPROM) [Tocolysis in the management of preterm premature rupture of membranes before 34 weeks of gestation: a double-blinded randomized controlled trial]. https://clinicaltrials.gov/show/nct03976063 (first received 2019).
IRCT20190819044568N1 {published data only}
-
- IRCT20190819044568N. Preterm labor inhibition. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20190819044568N1 (first received 2020).
IRCT20201017049052N1 {published data only}
-
- IRCT20201017049052N. Effect of magnesium sulfate and nifedipine in preterm labor. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20201017049052N1 (first received 2020).
NCT00466128 {published data only}
-
- NCT00466128. Indomethacin versus placebo in women with preterm premature rupture of membranes (PPROM). https://clinicaltrials.gov/show/NCT00466128 (first received 2007).
NCT01869361 {published data only}
-
- NCT01869361. Indomethacin for tocolysis. https://clinicaltrials.gov/show/NCT01869361 (first received 2013).
NCT02725736 {published data only}
-
- NCT02725736. Tocolytic therapy for preterm labor in multiple gestation. https://clinicaltrials.gov/show/NCT02725736 (first received 2016).
NCT03129945 {published data only}
-
- NCT03129945. Comparison of nifedipine versus indomethacin for acute preterm labor. https://clinicaltrials.gov/show/NCT03129945 (first received 2015).
NCT03298191 {published data only}
-
- NCT03298191. Tocolysis in prevention of preterm labor. https://clinicaltrials.gov/show/NCT03298191 (first received 2017).
NCT03542552 {published data only}
-
- NCT03542552. Nifedipine versus magnesium sulfate for prevention of preterm labor in symptomatic placenta previa. https://clinicaltrials.gov/show/NCT03542552 (first received 2018).
NCT04404686 {published data only}
-
- NCT04404686. Vaginal indomethacin for preterm labor. https://clinicaltrials.gov/show/NCT04404686 (first received 2020).
NCT04846621 {published data only}
-
- NCT04846621. Comparative study between nicorandil and nifedipine for the treatment of preterm labour. https://clinicaltrials.gov/show/NCT04846621 (first received 2021).
NTR6646 {published data only}
-
- NTR6646. Assessing the safety and effectiveness of tocolysis for preterm labour. http://www.who.int/trialsearch/Trial2.aspx?TrialID=NTR6646 (first received 2017).
PACTR202004681537890 {published data only}
-
- PACTR202004681537890. Prevention of premature birth by nifedipine alone or with indomethacin. http://www.who.int/trialsearch/Trial2.aspx?TrialID=PACTR202004681537890 (first received 2020).
TCTR20200617001 {published data only}
-
- TCTR20200617001. Effect of non-tocolytic drugs to delivery of pregnant women with threatened preterm labour and cervical length > 25 millimeters: a randomized controlled trial. http://www.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20200617001 (first received 2020).
Additional references
Babay 1998
-
- Babay Z, Lange IR, Amin H. Neonatal and maternal complications after administration of indomethacin for preterm labour between 24 and 30 weeks gestation. Journal of the Society of Obstetricians and Gynaecologists of Canada 1998;20:505-11.
Bain 2013
Blondel 2006
-
- Blondel B, Macfarlane A, Gissler M, Breart G, Zeitlin J, PERISTAT Study Group. Preterm birth and multiple pregnancy in European countries participating in the PERISTAT project. BJOG 2006;113:528-35. - PubMed
Brignardello‐Petersen 2018
-
- Brignardello-Petersen R, Bonner A, Alexander PE, Siemieniuk RA, Furukawa TA, Rochwerg B, et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. Journal of Clinical Epidemiology 2018;93:36-44. - PubMed
Caldwell 2005
Caldwell 2010
-
- Caldwell DM, Welton NJ, Ades AE. Mixed treatment comparison analysis provides internally coherent treatment effect estimates based on overviews of reviews and can reveal inconsistency. Journal of Clinical Epidemiology 2010;63(8):875-82. - PubMed
Carlisle 2017
-
- Carlisle JB. Data fabrication and other reasons for non-random sampling in 5087 randomised, controlled trials in anaesthetic and general medical journals. Anaesthesia 2017;72:944–52. - PubMed
Chaimani 2015
-
- Chaimani A, Salanti G. Visualizing assumptions and results in network meta-analysis: the network graphs package. Stata Journal 2015;15:4.
Crowther 2014
Deeks 2021
-
- Deeks JJ, Higgins JP, Altman DG editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:77–88. - PubMed
Duckitt 2014
Escobar 2006
Flenady 2014a
Flenady 2014b
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 3 January 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Haas 2009
-
- Haas DM, Imperiale TF, Kirkpatrick PR, Klein RW, Zollinger TW, Golichowski AM. Tocolytic therapy: a meta-analysis and decision analysis. Obstetrics and Gynecology 2009;113:585-94. - PubMed
Haas 2012
Higgins 2002
-
- Higgins JP. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. - PubMed
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2012
Higgins 2021
-
- Higgins JP, Eldridge S, Li T editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Kalra 2008
-
- Kalra SK, Molinaro TA. The association of in vitro fertilization and perinatal morbidity. Seminars in Reproductive Medicine 2008;26:423-35. - PubMed
Kinney 2006
-
- Kinney HC. The near-term (late preterm) human brain and risk for periventricular leukomalacia: a review. Seminars in Perinatology 2006;30:81–8. - PubMed
Koren 2006
-
- Koren G, Florescu A, Costei AM, Boskovic R, Moretti ME. Nonsteroidal anti-inflammatory drugs during third trimester and the risk of premature closure of the ductus arteriosus: a meta-analysis. Annals of Pharmacotherapy 2006;40:824-9. - PubMed
Lee 2008
-
- Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. American Journal of Obstetrics and Gynecology 2008;198:e1-8. - PubMed
Liberati 2009
Liu 2016
Medicines.org.uk 2020
-
- Medicinesorguk. Summary of Product Characteristics (SPC). www.medicines.org.uk/emc/ (accessed 2 December 2020).
Menon 2008
-
- Menon R. Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstetricia et Gynecologica Scandinavica 2008;87:590-600. - PubMed
Mukhopadhaya 2007
-
- Mukhopadhaya N, Arulkumaran S. Reproductive outcomes after in-vitro fertilization. Current Opinion in Obstetrics and Gynecology 2007;19:113-9. - PubMed
Neilson 2014
NICE 2015
-
- National Institute for Health and Clinical Excellence. Preterm labour and birth. NICE guideline [NG25]. https://www.nice.org.uk/guidance/ng25 accessed 3 January 2022.
Puhan 2014
-
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:5630. - PubMed
Reinebrant 2015
Review Manager 2020 [Computer program]
-
- Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Salanti 2011
-
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163-71. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.gradepro.org/app/handbook/handbook.html.
Schünemann 2021
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
StataCorp 2019 [Computer program]
-
- Stata Statistical Software: Release 16. StataCorp. CollegeStation, TX, 2019.
Su 2014
Turner 2012
UNIGME 2020
-
- United Nations Inter-agency Group for Child Mortality Estimation (UNIGME). Levels and trends in child mortality: report 2020. https://www.unicef.org/reports/levels-and-trends-child-mortality-report-... accessed 3 January 2022.
Wang 2004
-
- Wang ML, Dorer DJ, Fleming MP, Catlin EA. Clinical outcomes of near-term infants. Pediatrics 2004;114:372-6. - PubMed
White 2011
-
- White I. Multivariate random-effects meta-regression: Updates to mvmeta. Stata Journal 2011;11(2):255-70.
White 2015
-
- White IR. Network meta-analysis. Stata Journal 2015;15:4.
WHO 2015
-
- World Health Organization. WHO recommendations on interventions to improve preterm birth outcomes. https://apps.who.int/iris/bitstream/handle/10665/183037/9789241508988_en... accessed 3 January 2022. - PubMed
WHO 2018
-
- World Health Organization. Preterm birth: key facts. www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed 2 December 2020).
References to other published versions of this review
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

