Targets of autoantibodies in acquired hemophilia A are not restricted to factor VIII: data from the GTH-AH 01/2010 study
- PMID: 35947142
- PMCID: PMC9830154
- DOI: 10.1182/bloodadvances.2022008071
Targets of autoantibodies in acquired hemophilia A are not restricted to factor VIII: data from the GTH-AH 01/2010 study
Abstract
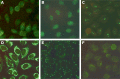
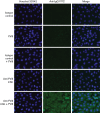
The root cause of autoantibody formation against factor VIII (FVIII) in acquired hemophilia A (AHA) remains unclear. We aimed to assess whether AHA is exclusively associated with autoantibodies toward FVIII or whether patients also produce increased levels of autoantibodies against other targets. A case-control study was performed enrolling patients with AHA and age-matched controls. Human epithelial cell (HEp-2) immunofluorescence was applied to screen for antinuclear (ANA) and anticytoplasmic autoantibodies. Screening for autoantibodies against extractable nuclear antigens was performed by enzyme immunoassay detecting SS-A/Ro, SS-B/La, U1RNP, Scl-70, Jo-1, centromere B, Sm, double-stranded DNA, and α-fodrin (AF). Patients with AHA were more often positive for ANA than control patients (64% vs 30%; odds ratio [OR] 4.02, 1.98-8.18) and had higher ANA titers detected than controls. Cytoplasmic autoantibodies and anti-AF immunoglobulin A autoantibodies were also more frequent in patients with AHA compared with controls. Autoantibodies against any target other than FVIII were found in 78% of patients with AHA compared with 46% of controls (OR 4.16, 1.98-8.39). Results were similar preforming sensitivity analyses (excluding either subjects with autoimmune disorders, cancer, pregnancy, or immunosuppressive medication at baseline) and in multivariable binary logistic regression. To exclude that autoantibody staining was merely a result of cross-reactivity of anti-FVIII autoantibodies, we tested a mix of 7 well-characterized monoclonal anti-FVIII antibodies. These antibodies did not stain HEp-2 cells used for ANA detection. In conclusion, a diverse pattern of autoantibodies is associated with AHA, suggesting that a more general breakdown of immune tolerance might be involved in its pathology.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: O.O. reports grants for research from Biotest and Octapharma outside the submitted work. S.W. reports grants for research from Biotest and Octapharma outside the submitted work. A.K. reports grants for research from Biotest and Octapharma outside the submitted work. T.W. reports fees for lectures from AbbVie, Amgen, AstraZeneca, BMS, Boehringer Ingelheim, Chugai, GSK, Lilly, Medac, Novartis, Pfizer, Roche, Sanofi, Takeda, UCB, and Viatris. H.E. reports grants for studies and research from Bayer, CSL Behring, and Pfizer and personal fees for lectures or consultancy from Bayer, BioMarin, Biotest, CSL Behring, Novo Nordisk, Pfizer, Roche, and SOBI. R.K. reports grants for studies and research from Bayer, Leo, and Takeda and personal fees for lectures or consultancy from Bayer, BioMarin, Biotest, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche/Chugai, Sanofi, SOBI, and Takeda outside the submitted work. K.H. reports grants for studies and research from Bayer, CSL Behring, Pfizer, Sobi, and Roche/Chugai outside the submitted work and personal fees for lectures and consultancy from Bayer, Biotest, Chugai, CSL Behring, LFB, Novo Nordisk, Pfizer, Roche, Sobi, and Takeda. C.H. reports travel grants and personal fees for lectures or consultancy from Bayer, BMS, CSL Behring, Daiichi Sankyo, Novo Nordisk, Octapharma, Pfizer, Roche/Chugai, Sanofi, SOBI, and Takeda outside the submitted work. C.P. reports grants for studies and research from Chugai/Roche, Leo Pharma, Zacros, and Takeda outside the submitted work and personal fees for lectures or consultancy from Bayer, BMS, Chugai/Roche, CSL Behring, Novo Nordisk, Pfizer, Sanofi, SOBI, and Takeda outside the submitted work. P.K. reports research grants, travel support, and consultancy fees from Roche, Novo Nordisk, Takeda, Octapharma, and Sanofi outside the submitted work. R.G. reports grants for research from Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, BMS, MSD, Sandoz, Gilead, Daiichi Sankyo, and AbbVie outside the submitted work; support for travel, accommodation, and expenses from Roche, Amgen, Janssen, AstraZeneca, Novartis, MSD, Celgene, Gilead, BMS, AbbVie, and Daiichi Sankyo outside the submitted work; and honoraria or consultancy fees from Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, BMS, MSD, Sandoz, AbbVie, Gilead, Daiichi Sankyo, Sanofi, and Janssen outside the submitted work. P.N. reports consultancy fees from Roche, Amgen, Novo Nordisk, Bayer, Sobi, and Takeda outside the submitted work. B.M.R. reports fees for consultancy from Takeda, Biomarin, Novo Nordisk, Boehringer Ingelheim, Fresenius Kabi, and Checkimmune outside the submitted work. A.T. reports grants for studies and research from Bayer, Biotest, Chugai/Roche, Novo Nordisk, Octapharma, Pfizer, and Takeda outside the submitted work and personal fees for lectures or consultancy from Bayer, Biotest, Chugai/Roche, CSL Behring, Novo Nordisk, Octapharma, Pfizer, SOBI, and Takeda outside the submitted work.
Figures


References
-
- Kruse-Jarres R, Kempton CL, Baudo F, et al. Acquired hemophilia A: updated review of evidence and treatment guidance. Am J Hematol. 2017;92(7):695–705. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

