Defective bone repletion in aged Balb/cBy mice was caused by impaired osteoblastic differentiation
- PMID: 35947191
- PMCID: PMC9722502
- DOI: 10.1007/s00774-022-01361-3
Defective bone repletion in aged Balb/cBy mice was caused by impaired osteoblastic differentiation
Abstract
Introduction: This study was undertaken to gain mechanistic information about bone repair using the bone repletion model in aged Balb/cBy mice.
Materials and methods: one month-old (young) mice were fed a calcium-deficient diet for 2 weeks and 8 month-old (adult) and 21-25 month-old (aged) female mice for 4 weeks during depletion, which was followed by feeding a calcium-sufficient diet for 16 days during repletion. To determine if prolonged repletion would improve bone repair, an additional group of aged mice were repleted for 4 additional weeks. Control mice were fed calcium-sufficient diet throughout. In vivo bone repletion response was assessed by bone mineral density gain and histomorphometry. In vitro response was monitored by osteoblastic proliferation, differentiation, and senescence.
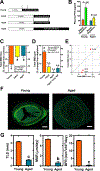
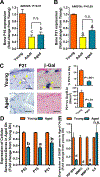
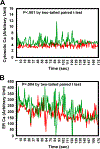
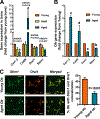
Results: There was no significant bone repletion in aged mice even with an extended repletion period, indicating an impaired bone repletion. This was not due to an increase in bone cell senescence or reduction in osteoblast proliferation, but to dysfunctional osteoblastic differentiation in aged bone cells. Osteoblasts of aged mice had elevated levels of cytosolic and ER calcium, which were associated with increased Cav1.2 and CaSR (extracellular calcium channels) expression but reduced expression of Orai1 and Stim1, key components of Stored Operated Ca2+ Entry (SOCE). Activation of Cav1.2 and CaSR leads to increased osteoblastic proliferation, but activation of SOCE is associated with osteoblastic differentiation.
Conclusion: The bone repletion mechanism in aged Balb/cBy mice is defective that is caused by an impaired osteoblast differentiation through reducedactivation of SOCE.
Keywords: Aging; Bone repletion; Calcium channels; Osteoblast differentiation; Stored operated calcium entry.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Conflicts of Interest Statement
All authors (Matilda H.-C. Sheng, Kin-Hing William Lau, Charles H. Rundle, Anar Alsunna, Sean M. Wilson, and David J. Baylink) have no conflict of interest to declare.
Figures
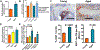

Similar articles
-
Synergistic effects of high dietary calcium and exogenous parathyroid hormone in promoting osteoblastic bone formation in mice.Br J Nutr. 2015 Mar 28;113(6):909-22. doi: 10.1017/S0007114514004309. Epub 2015 Mar 6. Br J Nutr. 2015. PMID: 25744000 Free PMC article.
-
Elevation of extracellular Ca2+ induces store-operated calcium entry via calcium-sensing receptors: a pathway contributes to the proliferation of osteoblasts.PLoS One. 2014 Sep 25;9(9):e107217. doi: 10.1371/journal.pone.0107217. eCollection 2014. PLoS One. 2014. PMID: 25254954 Free PMC article.
-
Unique Regenerative Mechanism to Replace Bone Lost During Dietary Bone Depletion in Weanling Mice.Endocrinology. 2017 Apr 1;158(4):714-729. doi: 10.1210/en.2016-1379. Endocrinology. 2017. PMID: 28324039
-
Marine algae possess therapeutic potential for Ca-mineralization via osteoblastic differentiation.Adv Food Nutr Res. 2011;64:429-41. doi: 10.1016/B978-0-12-387669-0.00033-8. Adv Food Nutr Res. 2011. PMID: 22054966 Review.
-
What role for store-operated Ca²⁺ entry in muscle?Microcirculation. 2013 May;20(4):330-6. doi: 10.1111/micc.12042. Microcirculation. 2013. PMID: 23312019 Free PMC article. Review.
Cited by
-
Microvesicles Released by Osteoclastic Cells Exhibited Chondrogenic, Osteogenic, and Anti-Inflammatory Activities: An Evaluation of the Feasibility of Their Use for Treatment of Osteoarthritis in a Mouse Model.Cells. 2025 Jan 28;14(3):193. doi: 10.3390/cells14030193. Cells. 2025. PMID: 39936984 Free PMC article.
References
-
- Gudmundsdottir SL, Indridason OS, Franzson L, Sigurdsson G (2005) Age-related decline in bone mass measured by dual-energy X-ray absorptiometry and quantitative ultrasound in a population-based sample of both sexes: identification of useful ultrasound thresholds for osteoporosis screening. J Clin Densitom 8:80–6 doi:10.1385/jcd:8:1:080 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

