Thyroid hormone signaling in the intestinal stem cells and their niche
- PMID: 35947210
- PMCID: PMC11072102
- DOI: 10.1007/s00018-022-04503-y
Thyroid hormone signaling in the intestinal stem cells and their niche
Abstract
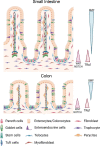
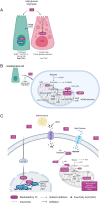
Several studies emphasized the function of the thyroid hormones in stem cell biology. These hormones act through the nuclear hormone receptor TRs, which are T3-modulated transcription factors. Pioneer work on T3-dependent amphibian metamorphosis showed that the crosstalk between the epithelium and the underlying mesenchyme is absolutely required for intestinal maturation and stem cell emergence. With the recent advances of powerful animal models and 3D-organoid cultures, similar findings have now begun to be described in mammals, where the action of T3 and TRα1 control physiological and cancer-related stem cell biology. In this review, we have summarized recent findings on the multiple functions of T3 and TRα1 in intestinal epithelium stem cells, cancer stem cells and their niche. In particular, we have highlighted the regulation of metabolic functions directly linked to normal and/or cancer stem cell biology. These findings help explain other possible mechanisms by which TRα1 controls stem cell biology, beyond the more classical Wnt and Notch signaling pathways.
Keywords: Colon cancer; Intestinal epithelium; Stem cell; Stem cell niche; Thyroid hormone; Thyroid hormone receptor.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

