Flow cytometry-based viability staining: an at-line tool for bioprocess monitoring of Sulfolobus acidocaldarius
- PMID: 35947320
- PMCID: PMC9365904
- DOI: 10.1186/s13568-022-01447-1
Flow cytometry-based viability staining: an at-line tool for bioprocess monitoring of Sulfolobus acidocaldarius
Abstract
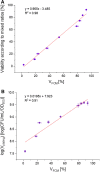
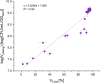
Determination of the viability, ratio of dead and live cell populations, of Sulfolobus acidocaldarius is still being done by tedious and material-intensive plating assays that can only provide time-lagged results. Although S. acidocaldarius, an extremophilic Archaeon thriving at 75 °C and pH 3.0, and related species harbor great potential for the exploitation as production hosts and biocatalysts in biotechnological applications, no industrial processes have been established yet. One hindrance is that during development and scaling of industrial bioprocesses timely monitoring of the impact of process parameters on the cultivated organism is crucial-a task that cannot be fulfilled by traditional plating assays. As alternative, flow cytometry (FCM) promises a fast and reliable method for viability assessment via the use of fluorescent dyes. In this study, commercially available fluorescent dyes applicable in S. acidocaldarius were identified. The dyes, fluorescein diacetate and concanavalin A conjugated with rhodamine, were discovered to be suitable for viability determination via FCM. For showing the applicability of the developed at-line tool for bioprocess monitoring, a chemostat cultivation on a defined growth medium at 75 °C, pH 3.0 was conducted. Over the timeframe of 800 h, this developed FCM method was compared to the plating assay by monitoring the change in viability upon controlled pH shifts. Both methods detected an impact on the viability at pH values of 2.0 and 1.5 when compared to pH 3.0. A logarithmic relationship between the viability observed via plating assay and via FCM was observed.
Keywords: Flow cytometry; Fluorescent dyes; Live/dead staining; Sulfolobus acidocaldarius; Viability.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest. D.J.W. and J.Q. disclose their employment at the company NovoArc GmbH which is engaged in lipid research and commercialization of archaeal lipids.
Figures


References
-
- Anemüller S, Lübben M, Schäfer G. The respiratory system of Sulfolobus acidocaldarius, a thermoacidophilic archaebacterium. FEBS Lett. 1985;193:83–87. doi: 10.1016/0014-5793(85)80084-3. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

