Biochemical and Biophysical Characterization of Carbonic Anhydrase VI from Human Milk and Saliva
- PMID: 35947329
- PMCID: PMC9464147
- DOI: 10.1007/s10930-022-10070-9
Biochemical and Biophysical Characterization of Carbonic Anhydrase VI from Human Milk and Saliva
Abstract
Carbonic anhydrases (CA, EC 4.2.1.1) catalyze the hydration of carbon dioxide and take part in many essential physiological processes. In humans, 15 CAs are characterized, including the only secreted isoenzyme CA VI. CA VI has been linked to specific processes in the mouth, namely bitter taste perception, dental caries, and maintenance of enamel pellicle, and implicated in several immunity-related phenomena. However, little is known of the mechanisms of the above. In this study, we characterized human CA VI purified from saliva and milk with biophysical methods and measured their enzyme activities and acetazolamide inhibition. Size-exclusion chromatography showed peaks of salivary and milk CA VI corresponding to hexameric state or larger at pH 7.5. At pH 5.0 the hexamer peaks dominated. SDS- PAGE of milk CA VI protein treated with a bifunctional crosslinker further confirmed that a majority of CA VI is oligomers of similar sizes in solution. Mass spectrometry experiments confirmed that both of the two putative N-glycosylation sites, Asn67 and Asn256, are heterogeneously glycosylated. The attached glycans in milk CA VI were di- and triantennary complex-type glycans, carrying both a core fucose and 1 to 2 additional fucose units, whereas the glycans in salivary CA VI were smaller, seemingly degraded forms of core fucosylated complex- or hybrid-type glycans. Mass spectrometry also verified the predicted signal peptide cleavage site and the terminal residue, Gln 18, being in pyroglutamate form. Thorough characterization of CA VI paves way to better understanding of the biological function of the protein.
Keywords: CA VI; CA6; Glycosylation; Mass spectrometry; Oligomerization; Size exclusion chromatography.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest. The authors declare that the data supporting the findings of this study are available within the article and its supplementary information files.
Figures
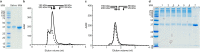





Similar articles
-
Salivary carbonic anhydrase isoenzyme VI is located in the human enamel pellicle.Caries Res. 1999 May-Jun;33(3):185-90. doi: 10.1159/000016515. Caries Res. 1999. PMID: 10207193
-
The identification of secreted carbonic anhydrase VI as a constitutive glycoprotein of human and rat milk.Proc Natl Acad Sci U S A. 2001 Sep 25;98(20):11604-8. doi: 10.1073/pnas.121172598. Epub 2001 Sep 11. Proc Natl Acad Sci U S A. 2001. PMID: 11553764 Free PMC article.
-
A low concentration of carbonic anhydrase isoenzyme VI in whole saliva is associated with caries prevalence.Caries Res. 1999 May-Jun;33(3):178-84. doi: 10.1159/000016514. Caries Res. 1999. PMID: 10207192
-
Salivary carbonic anhydrase isoenzyme VI.J Physiol. 1999 Oct 15;520 Pt 2(Pt 2):315-20. doi: 10.1111/j.1469-7793.1999.t01-1-00315.x. J Physiol. 1999. PMID: 10523402 Free PMC article. Review.
-
Carbonic anhydrases as drug targets--an overview.Curr Top Med Chem. 2007;7(9):825-33. doi: 10.2174/156802607780636690. Curr Top Med Chem. 2007. PMID: 17504127 Review.
Cited by
-
Changes in the Protein Profile of Saliva from People with Obesity Treated with Bariatric Surgery and Physical Exercise.Int J Mol Sci. 2025 Jun 12;26(12):5622. doi: 10.3390/ijms26125622. Int J Mol Sci. 2025. PMID: 40565086 Free PMC article.
-
Lasamide, a Potent Human Carbonic Anhydrase Inhibitor from the Market: Inhibition Profiling and Crystallographic Studies.ACS Med Chem Lett. 2024 Sep 30;15(10):1749-1755. doi: 10.1021/acsmedchemlett.4c00341. eCollection 2024 Oct 10. ACS Med Chem Lett. 2024. PMID: 39411526
-
Human Milk Feeding in Inherited Metabolic Disorders: A Systematic Review of Growth, Metabolic Control, and Neurodevelopment Outcomes.J Inherit Metab Dis. 2025 Mar;48(2):e70001. doi: 10.1002/jimd.70001. J Inherit Metab Dis. 2025. PMID: 39912448 Free PMC article.
-
The Secret of Secrets: Carbonic Anhydrase Concentration in Lizards' Femoral Gland Secretions Is Tuned to Environmental Conditions.Ecol Evol. 2025 Aug 21;15(8):e72023. doi: 10.1002/ece3.72023. eCollection 2025 Aug. Ecol Evol. 2025. PMID: 40852626 Free PMC article.
References
-
- Chegwidden WR, Carter ND. Introduction to the carbonic anhydrases. EXS. 2000;90:14–28. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

