Treatment Patterns for Targeted Therapies, Non-Targeted Therapies, and Drug Holidays in Patients with Psoriasis
- PMID: 35947341
- PMCID: PMC9464286
- DOI: 10.1007/s13555-022-00775-1
Treatment Patterns for Targeted Therapies, Non-Targeted Therapies, and Drug Holidays in Patients with Psoriasis
Abstract
Introduction: We aimed to evaluate US treatment patterns and, more specifically, switch patterns among patients with psoriasis (PsO) who initiated treatment with targeted therapy (TT) and subsequently switched to another therapy.
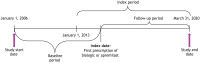
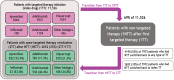
Methods: This retrospective study used IBM® MarketScan® Commercial and Medicare Databases (1/1/2006-3/31/2020) to evaluate treatment patterns in biologic- and apremilast-naive patients with PsO. TT included apremilast, adalimumab, etanercept, infliximab, ustekinumab, or other biologics (certolizumab pegol, secukinumab, brodalumab, ixekizumab, guselkumab, or tildrakizumab). Adults with ≥ 1 prescription for a TT, ≥ 2 PsO claims separated by ≥ 1 day on or before the index date (date of first TT prescription), and continuous medical and pharmacy enrollment for 1 year before and 2 years after the index date were eligible. Non-targeted therapy (NTT) was defined as non-targeted oral systemic treatment, topical treatment, phototherapy, or no treatment. Kaplan-Meier (KM) analysis was used to estimate time to reinitiation of TT (24-month continuous enrollment post-index was not required).
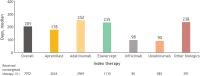
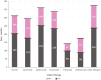
Results: A total of 11,526 patients with PsO were included; mean [standard deviation (SD)] age and Charlson Comorbidity Index score were 48.3 (12.8) years and 0.9 (1.43), respectively. During the follow-up, 69.2% of the patients were treated with NTT. Median time to first NTT, for those who received NTT, was 205 days (longest: adalimumab, 252 days). Among patients who switched to NTT after initiating treatment with TT, 52.6% reinitiated treatment with TT (least common: apremilast, 45.6%), with a median time to reinitiation of 106 days (longest: other biologics, 136 days). For all patients on NTT, the probability of reinitiating any TT was 60.7% at 24 months.
Conclusions: PsO treatment is often cyclical in nature. Patients frequently experience drug holidays or transition back to TT after using NTT. The consideration of real-world treatment patterns in future economic models may provide new insights into the clinical effectiveness and value of PsO treatments.
Keywords: Biologics; Epidemiology; IL inhibitor; Observational study; PDE-4 inhibitor; Psoriasis; TNF inhibitor; Treatment switch.
Plain language summary
Psoriasis is a chronic inflammatory skin disease that affects 3.0% of adults or an estimated 7.56 million Americans. The most common type of psoriasis is called plaque psoriasis because of its appearance with red patches and silvery scales on the skin. A major concern of medical providers is that not all patients continue their treatment as prescribed. Many patients discontinue, switch, and often restart treatment. To develop effective psoriasis treatment plans for shared decision-making among medical providers and patients, it is important to look at how treatments are used in the real world. This can be done by conducting studies using insurance claims data from healthcare insurance providers. In this study, we evaluated treatment patterns and, more specifically, patterns in changes of treatment in US patients who began their psoriasis treatment with a targeted therapy (biologics or apremilast) and then changed to another therapy. We found that patients often took drug holidays (days with no treatment) and returned back to using a targeted therapy after using a non-targeted therapy (e.g., other oral therapy, topical treatment, phototherapy, or no treatment). Findings from this real-world study may support future studies on the clinical effectiveness and value of current and future treatments for psoriasis—especially within these targeted to non-targeted transitions.
© 2022. The Author(s).
Figures




References
-
- Institute for Clinical and Economic Review. Targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: effectiveness and value. Condition update (final evidence report). Boston: Institute for Clinical and Economic Review; 2018.
LinkOut - more resources
Full Text Sources
Research Materials

