Assessment of the Diagnostic Yield of Combined Cardiomyopathy and Arrhythmia Genetic Testing
- PMID: 35947370
- PMCID: PMC9366660
- DOI: 10.1001/jamacardio.2022.2455
Assessment of the Diagnostic Yield of Combined Cardiomyopathy and Arrhythmia Genetic Testing
Abstract
Importance: Genetic testing can guide management of both cardiomyopathies and arrhythmias, but cost, yield, and uncertain results can be barriers to its use. It is unknown whether combined disease testing can improve diagnostic yield and clinical utility for patients with a suspected genetic cardiomyopathy or arrhythmia.
Objective: To evaluate the diagnostic yield and clinical management implications of combined cardiomyopathy and arrhythmia genetic testing through a no-charge, sponsored program for patients with a suspected genetic cardiomyopathy or arrhythmia.
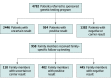
Design, setting, and participants: This cohort study involved a retrospective review of DNA sequencing results for cardiomyopathy- and arrhythmia-associated genes. The study included 4782 patients with a suspected genetic cardiomyopathy or arrhythmia who were referred for genetic testing by 1203 clinicians; all patients participated in a no-charge, sponsored genetic testing program for cases of suspected genetic cardiomyopathy and arrhythmia at a single testing site from July 12, 2019, through July 9, 2020.
Main outcomes and measures: Positive gene findings from combined cardiomyopathy and arrhythmia testing were compared with findings from smaller subtype-specific gene panels and clinician-provided diagnoses.
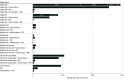
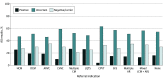
Results: Among 4782 patients (mean [SD] age, 40.5 [21.3] years; 2551 male [53.3%]) who received genetic testing, 39 patients (0.8%) were Ashkenazi Jewish, 113 (2.4%) were Asian, 571 (11.9%) were Black or African American, 375 (7.8%) were Hispanic, 2866 (59.9%) were White, 240 (5.0%) were of multiple races and/or ethnicities, 138 (2.9%) were of other races and/or ethnicities, and 440 (9.2%) were of unknown race and/or ethnicity. A positive result (molecular diagnosis) was confirmed in 954 of 4782 patients (19.9%). Of those, 630 patients with positive results (66.0%) had the potential to inform clinical management associated with adverse clinical outcomes, increased arrhythmia risk, or targeted therapies. Combined cardiomyopathy and arrhythmia gene panel testing identified clinically relevant variants for 1 in 5 patients suspected of having a genetic cardiomyopathy or arrhythmia. If only patients with a high suspicion of genetic cardiomyopathy or arrhythmia had been tested, at least 137 positive results (14.4%) would have been missed. If testing had been restricted to panels associated with the clinician-provided diagnostic indications, 75 of 689 positive results (10.9%) would have been missed; 27 of 75 findings (36.0%) gained through combined testing involved a cardiomyopathy indication with an arrhythmia genetic finding or vice versa. Cascade testing of family members yielded 402 of 958 positive results (42.0%). Overall, 2446 of 4782 patients (51.2%) had only variants of uncertain significance. Patients referred for arrhythmogenic cardiomyopathy had the lowest rate of variants of uncertain significance (81 of 176 patients [46.0%]), and patients referred for catecholaminergic polymorphic ventricular tachycardia had the highest rate (48 of 76 patients [63.2%]).
Conclusions and relevance: In this study, comprehensive genetic testing for cardiomyopathies and arrhythmias revealed diagnoses that would have been missed by disease-specific testing. In addition, comprehensive testing provided diagnostic and prognostic information that could have potentially changed management and monitoring strategies for patients and their family members. These results suggest that this improved diagnostic yield may outweigh the burden of uncertain results.
Conflict of interest statement
Figures
Comment in
-
Genetic Testing Panels in Inherited Cardiac Diseases-Does Size Really Matter?JAMA Cardiol. 2022 Sep 1;7(9):889-890. doi: 10.1001/jamacardio.2022.2465. JAMA Cardiol. 2022. PMID: 35947367 No abstract available.
References
-
- Ackerman MJ, Priori SG, Willems S, et al. . HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 2011;8(8):1308-1339. doi:10.1016/j.hrthm.2011.05.020 - DOI - PubMed
-
- Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. . 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018;138(13):e272-e391. doi:10.1161/CIR.0000000000000549 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

