Pharmacology, particle deposition and drug administration techniques of intranasal corticosteroids for treating allergic rhinitis
- PMID: 35947495
- PMCID: PMC9804774
- DOI: 10.1111/cea.14212
Pharmacology, particle deposition and drug administration techniques of intranasal corticosteroids for treating allergic rhinitis
Abstract
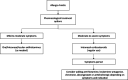
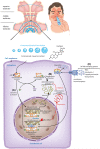
This review presents an overview of the available literature regarding intranasal corticosteroids (INCs) for the treatment of allergic rhinitis (AR). Various treatment options exist for AR including INCs, antihistamines and leucotriene antagonists. INCs are considered to be the most effective therapy for moderate-to-severe AR, as they are effective against nasal and ocular symptoms and improve quality of life. Their safety has been widely observed. INCs are effective and safe for short-term use. Local adverse events are observed but generally well-tolerated. The occurrence of (serious) systemic adverse events is unlikely but cannot be ruled out. There is a lack of long-term safety data. INC may cause serious eye complications. The risk of INCs on the hypothalamic-pituitary-adrenal axis, on bone mineral density reduction or osteoporosis and on growth in children, should be considered during treatment. Pharmacological characteristics of INCs (e.g. the mode of action and pharmacokinetics) are well known and described. We sought to gain insight into whether specific properties affect the efficacy and safety of INCs, including nasal particle deposition, which the administration technique affects. However, advances are lacking regarding the improved understanding of the effect of particle deposition on efficacy and safety and the effect of the administration technique. This review emphasizes the gaps in knowledge regarding this subject. Advances in research and health care are necessary to improve care for patients with AR.
© 2022 The Authors. Clinical & Experimental Allergy published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no relevant conflicts of interest.
Figures

References
-
- Roberts G, Xatzipsalti M, Borrego LM, et al. Paediatric rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy. 2013;68(9):1102‐1116. - PubMed
-
- Bousquet PJ, Leynaert B, Neukirch F, et al. Geographical distribution of atopic rhinitis in the European Community Respiratory Health Survey I. Allergy. 2008;63:1301‐1309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

