Chaperone requirements for de novo folding of Saccharomyces cerevisiae septins
- PMID: 35947497
- PMCID: PMC9635297
- DOI: 10.1091/mbc.E22-07-0262
Chaperone requirements for de novo folding of Saccharomyces cerevisiae septins
Abstract
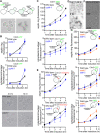
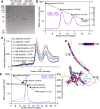
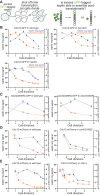
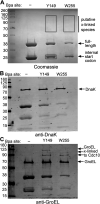
Polymers of septin protein complexes play cytoskeletal roles in eukaryotic cells. The specific subunit composition within complexes controls functions and higher-order structural properties. All septins have globular GTPase domains. The other eukaryotic cytoskeletal NTPases strictly require assistance from molecular chaperones of the cytosol, particularly the cage-like chaperonins, to fold into oligomerization-competent conformations. We previously identified cytosolic chaperones that bind septins and influence the oligomerization ability of septins carrying mutations linked to human disease, but it was unknown to what extent wild-type septins require chaperone assistance for their native folding. Here we use a combination of in vivo and in vitro approaches to demonstrate chaperone requirements for de novo folding and complex assembly by budding yeast septins. Individually purified septins adopted nonnative conformations and formed nonnative homodimers. In chaperonin- or Hsp70-deficient cells, septins folded slower and were unable to assemble posttranslationally into native complexes. One septin, Cdc12, was so dependent on cotranslational chaperonin assistance that translation failed without it. Our findings point to distinct translation elongation rates for different septins as a possible mechanism to direct a stepwise, cotranslational assembly pathway in which general cytosolic chaperones act as key intermediaries.
Figures


References
-
- Albanèse V, Yam AY-W, Baughman J, Parnot C, Frydman J (2006). Systems analyses reveal two chaperone networks with distinct functions in eukaryotic cells. Cell 124, 75–88. - PubMed
-
- Amberg D, Burke D, Strathern J (2005). Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual, 2005 ed, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

