Plasticity in astrocyte subpopulations regulates heroin relapse
- PMID: 35947652
- PMCID: PMC9365285
- DOI: 10.1126/sciadv.abo7044
Plasticity in astrocyte subpopulations regulates heroin relapse
Abstract
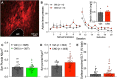
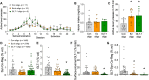
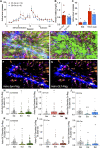
Opioid use disorder (OUD) produces detrimental personal and societal consequences. Astrocytes are a major cell group in the brain that receives little attention in mediating OUD. We determined how astrocytes and the astroglial glutamate transporter, GLT-1, in the nucleus accumbens core adapt and contribute to heroin seeking in rats. Seeking heroin, but not sucrose, produced two transient forms of plasticity in different astroglial subpopulations. Increased morphological proximity to synapses occurred in one subpopulation and increased extrasynaptic GLT-1 expression in another. Augmented synapse proximity by astroglia occurred selectively at D2-dopamine receptor-expressing dendrites, while changes in GLT-1 were not neuron subtype specific. mRNA-targeted antisense inhibition of either morphological or GLT-1 plasticity promoted cue-induced heroin seeking. Thus, we show that heroin cues induce two distinct forms of transient plasticity in separate astroglial subpopulations that dampen heroin relapse.
Figures



References
-
- Kalivas P. W., The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 10, 561–572 (2009). - PubMed
-
- Danbolt N. C., Glutamate uptake. Prog. Neurobiol. 65, 1–105 (2001). - PubMed
-
- Scofield M. D., Li H., Siemsen B. M., Healey K. L., Tran P. K., Woronoff N., Boger H. A., Kalivas P. W., Reissner K. J., Cocaine self-administration and extinction leads to reduced glial fibrillary acidic protein expression and morphometric features of astrocytes in the nucleus accumbens core. Biol. Psychiatry 80, 207–215 (2016). - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

