Biochemical and structural characterization of hepatitis A virus 2C reveals an unusual ribonuclease activity on single-stranded RNA
- PMID: 35947700
- PMCID: PMC9458454
- DOI: 10.1093/nar/gkac671
Biochemical and structural characterization of hepatitis A virus 2C reveals an unusual ribonuclease activity on single-stranded RNA
Abstract
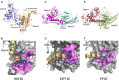
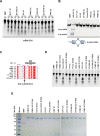
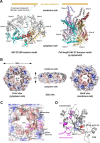
The HAV nonstructural protein 2C is essential for virus replication; however, its precise function remains elusive. Although HAV 2C shares 24-27% sequence identity with other 2Cs, key motifs are conserved. Here, we demonstrate that HAV 2C is an ATPase but lacking helicase activity. We identified an ATPase-independent nuclease activity of HAV 2C with a preference for polyuridylic single-stranded RNAs. We determined the crystal structure of an HAV 2C fragment to 2.2 Å resolution, containing an ATPase domain, a region equivalent to enterovirus 2C zinc-finger (ZFER) and a C-terminal amphipathic helix (PBD). The PBD of HAV 2C occupies a hydrophobic pocket (Pocket) in the adjacent 2C, and we show the PBD-Pocket interaction is vital for 2C functions. We identified acidic residues that are essential for the ribonuclease activity and demonstrated mutations at these sites abrogate virus replication. We built a hexameric-ring model of HAV 2C, revealing the ribonuclease-essential residues clustering around the central pore of the ring, whereas the ATPase active sites line up at the gaps between adjacent 2Cs. Finally, we show the ribonuclease activity is shared by other picornavirus 2Cs. Our findings identified a previously unfound activity of picornavirus 2C, providing novel insights into the mechanisms of virus replication.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





Similar articles
-
A cardioviral 2C-ATP complex structure reveals the essential role of a conserved arginine in regulation of cardioviral 2C activity.J Virol. 2024 Oct 22;98(10):e0091124. doi: 10.1128/jvi.00911-24. Epub 2024 Sep 6. J Virol. 2024. PMID: 39240112 Free PMC article.
-
An anti-picornaviral strategy based on the crystal structure of foot-and-mouth disease virus 2C protein.Cell Rep. 2022 Jul 5;40(1):111030. doi: 10.1016/j.celrep.2022.111030. Cell Rep. 2022. PMID: 35793627
-
Crystal structure of a soluble fragment of poliovirus 2CATPase.PLoS Pathog. 2018 Sep 19;14(9):e1007304. doi: 10.1371/journal.ppat.1007304. eCollection 2018 Sep. PLoS Pathog. 2018. PMID: 30231078 Free PMC article.
-
Picornavirus 2C proteins: structure-function relationships and interactions with host factors.Front Cell Infect Microbiol. 2024 Feb 23;14:1347615. doi: 10.3389/fcimb.2024.1347615. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38465233 Free PMC article. Review.
-
Picornavirus non-structural proteins as targets for new anti-virals with broad activity.Antiviral Res. 2011 Mar;89(3):204-18. doi: 10.1016/j.antiviral.2010.12.007. Epub 2011 Jan 12. Antiviral Res. 2011. PMID: 21236302 Review.
Cited by
-
Enzymatic characterization and dominant sites of foot-and-mouth disease virus 2C protein.Heliyon. 2024 Jul 30;10(15):e35449. doi: 10.1016/j.heliyon.2024.e35449. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39170175 Free PMC article.
-
Identification of Mulberrofuran as a potent inhibitor of hepatitis A virus 3Cpro and RdRP enzymes through structure-based virtual screening, dynamics simulation, and DFT studies.Mol Divers. 2024 Jun;28(3):1609-1628. doi: 10.1007/s11030-023-10679-7. Epub 2023 Jun 29. Mol Divers. 2024. PMID: 37386350
-
A cardioviral 2C-ATP complex structure reveals the essential role of a conserved arginine in regulation of cardioviral 2C activity.J Virol. 2024 Oct 22;98(10):e0091124. doi: 10.1128/jvi.00911-24. Epub 2024 Sep 6. J Virol. 2024. PMID: 39240112 Free PMC article.
-
Loxapine inhibits replication of hepatitis A virus in vitro and in vivo by targeting viral protein 2C.PLoS Pathog. 2024 Mar 13;20(3):e1012091. doi: 10.1371/journal.ppat.1012091. eCollection 2024 Mar. PLoS Pathog. 2024. PMID: 38478584 Free PMC article.
-
Leucoverdazyls as Novel Potent Inhibitors of Enterovirus Replication.Pathogens. 2024 May 15;13(5):410. doi: 10.3390/pathogens13050410. Pathogens. 2024. PMID: 38787262 Free PMC article.
References
-
- International Committee on Taxonomy of Viruses Virus Taxonomy: Classification and Nomenclature of Viruses: Sixth Report of the International Committee on Taxonomy of Viruses. 1995; NY: Springer-Verlag Wien Austria.
-
- Lemon S.M., Ott J.J., Van Damme P., Shouval D.. Type a viral hepatitis: a summary and update on the molecular virology, epidemiology, pathogenesis and prevention. J. Hepatol. 2017; 68:167–184. - PubMed
-
- Averhoff F.M., Khudyakov Y., Nelson N.P.. Plotkin S.A., Orenstein W.A., Offit P.A., Edwards K.M.. 24 - Hepatitis A Vaccines. Plotkin's Vaccines. 7th edn. 2018; Elsevier; 319–341.
-
- Hu X., Collier M.G., Xu F.. Hepatitis a outbreaks in developed countries: detection, control, and prevention. Foodborne Pathog Dis. 2020; 17:166–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

