Surface-modified, dye-sensitized niobate nanosheets enabling an efficient solar-driven Z-scheme for overall water splitting
- PMID: 35947708
- PMCID: PMC9365272
- DOI: 10.1126/sciadv.adc9115
Surface-modified, dye-sensitized niobate nanosheets enabling an efficient solar-driven Z-scheme for overall water splitting
Abstract
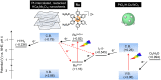
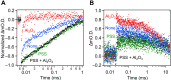
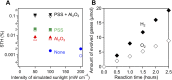
While dye-sensitized metal oxides are good candidates as H2 evolution photocatalysts for solar-driven Z-scheme water splitting, their solar-to-hydrogen (STH) energy conversion efficiencies remain low because of uncontrolled charge recombination reactions. Here, we show that modification of Ru dye-sensitized, Pt-intercalated HCa2Nb3O10 nanosheets (Ru/Pt/HCa2Nb3O10) with both amorphous Al2O3 and poly(styrenesulfonate) (PSS) improves the STH efficiency of Z-scheme overall water splitting by a factor of ~100, when the nanosheets are used in combination with a WO3-based O2 evolution photocatalyst and an I3-/I- redox mediator, relative to an analogous system that uses unmodified Ru/Pt/HCa2Nb3O10. By using the optimized photocatalyst, PSS/Ru/Al2O3/Pt/HCa2Nb3O10, a maximum STH of 0.12% and an apparent quantum yield of 4.1% at 420 nm were obtained, by far the highest among dye-sensitized water splitting systems and comparable to conventional semiconductor-based suspended particulate photocatalyst systems.
Figures

Similar articles
-
An Artificial Z-Scheme Constructed from Dye-Sensitized Metal Oxide Nanosheets for Visible Light-Driven Overall Water Splitting.J Am Chem Soc. 2020 May 6;142(18):8412-8420. doi: 10.1021/jacs.0c02053. Epub 2020 Apr 27. J Am Chem Soc. 2020. PMID: 32282192
-
Metal-free carbazole-thiophene photosensitizers designed for a dye-sensitized H2-evolving photocatalyst in Z-scheme water splitting.J Chem Phys. 2024 Jan 28;160(4):044710. doi: 10.1063/5.0179225. J Chem Phys. 2024. PMID: 38294313
-
Visible-light-induced water splitting based on two-step photoexcitation between dye-sensitized layered niobate and tungsten oxide photocatalysts in the presence of a triiodide/iodide shuttle redox mediator.J Am Chem Soc. 2013 Nov 13;135(45):16872-84. doi: 10.1021/ja4048637. Epub 2013 Oct 29. J Am Chem Soc. 2013. PMID: 24128384
-
Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting.Chem Soc Rev. 2019 Apr 1;48(7):2109-2125. doi: 10.1039/c8cs00542g. Chem Soc Rev. 2019. PMID: 30328438 Review.
-
Water Splitting on Rutile TiO2 -Based Photocatalysts.Chemistry. 2018 Dec 10;24(69):18204-18219. doi: 10.1002/chem.201800799. Epub 2018 Jun 6. Chemistry. 2018. PMID: 29570871 Review.
Cited by
-
Steering artificial photosynthesis via photoinduced conversion of monometallic to bimetallic sites in FeCo nitroprussides.Nat Commun. 2025 Jul 4;16(1):6160. doi: 10.1038/s41467-025-61129-x. Nat Commun. 2025. PMID: 40615385 Free PMC article.
-
Efficient Hydrogen Production by a Photoredox Cascade Catalyst Comprising Dual Photosensitizers and a Transparent Electron Mediator.J Am Chem Soc. 2023 Mar 22;145(11):6035-6038. doi: 10.1021/jacs.2c13687. Epub 2023 Mar 13. J Am Chem Soc. 2023. PMID: 36912645 Free PMC article.
-
Strain-induced charge delocalization achieves ultralow exciton binding energy toward efficient photocatalysis.Chem Sci. 2024 Nov 4;15(46):19546-19555. doi: 10.1039/d4sc05873a. eCollection 2024 Nov 27. Chem Sci. 2024. PMID: 39568926 Free PMC article.
-
Surface Modifications of Layered Perovskite Oxysulfide Photocatalyst Y2Ti2O5S2 to Enhance Visible-Light-Driven Water Splitting.Adv Sci (Weinh). 2025 Jan;12(3):e2412326. doi: 10.1002/advs.202412326. Epub 2024 Nov 27. Adv Sci (Weinh). 2025. PMID: 39601320 Free PMC article.
-
Highly Efficient Liquid-Phase Exfoliation of Layered Perovskite-like Titanates HLnTiO4 and H2Ln2Ti3O10 (Ln = La, Nd) into Nanosheets.Nanomaterials (Basel). 2023 Nov 29;13(23):3052. doi: 10.3390/nano13233052. Nanomaterials (Basel). 2023. PMID: 38063747 Free PMC article.
References
-
- Maeda K., Domen K., Photocatalytic water splitting: Recent progress and future challenges. J. Phys. Chem. Lett. 1, 2655–2661 (2010).
-
- Nishiyama H., Yamada T., Nakabayashi M., Maehara Y., Yamaguchi M., Kuromiya Y., Nagatsuma Y., Tokudome H., Akiyama S., Watanabe T., Narushima R., Okunaka S., Shibata N., Takata T., Hisatomi T., Domen K., Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 598, 304–307 (2021). - PubMed
-
- Youngblood W. J., Lee S.-H. A., Maeda K., Mallouk T. E., Visible light water splitting using dye-sensitized oxide semiconductors. Acc. Chem. Res. 42, 1966–1973 (2009). - PubMed
-
- Abe R., Development of a new system for photocatalytic water splitting into H2 and O2 under visible light irradiation. Bull. Chem. Soc. Jpn. 84, 1000–1030 (2011).
-
- Nakada A., Kumagai H., Robert M., Ishitani O., Maeda K., Molecule/semiconductor hybrid materials for visible-light CO2 reduction: Design principles and interfacial engineering. Acc. Mater. Res. 2, 458–470 (2021).
LinkOut - more resources
Full Text Sources

