Do studies published in two leading reproduction journals between 2011 and 2020 demonstrate that they followed WHO5 recommendations for basic semen analysis?
- PMID: 35947767
- PMCID: PMC9527455
- DOI: 10.1093/humrep/deac173
Do studies published in two leading reproduction journals between 2011 and 2020 demonstrate that they followed WHO5 recommendations for basic semen analysis?
Abstract
Study question: Do publications that involve the interpretation of the results of a basic semen analysis, published in Human Reproduction and Fertility & Sterility between 2011 and 2020, give sufficient evidence in their methodology to demonstrate that they followed the technical methods recommended in the fifth edition of the World Health Organization (WHO) laboratory manual, entitled WHO Laboratory Manual for the Examination and Processing of Human Semen (WHO5)?
Summary answer: Evidence of methodological agreement of studies with the WHO5 recommendations was low, despite 70% of papers stating that they followed WHO5 recommendations.
What is known already: A basic semen analysis is currently an integral part of infertility investigations of the male, but method standardization in laboratories remains an issue. The different editions of the WHO manual for the basic semen analysis (WHO1-6) have attempted to address this by providing increasingly rigorous methodological protocols to reduce experimental error. However, to what extent these methods are followed by studies that involve the interpretation of the results of basic semen analysis remains unknown.
Study design, size, duration: A survey of the technical methods used to perform a basic semen analysis was conducted on studies published in two leading reproduction journals (Human Reproduction and Fertility & Sterility) between 2011 and 2020.
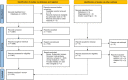
Participants/materials, setting, methods: The literature search was performed on the electronic databases PUBMED and MEDLINE Ovid between January 2021 and March 2021. The MeSH terms included in the search were 'sperm concentration' OR 'sperm motility' OR 'sperm morphology' OR 'sperm vitality' OR 'male fertility' AND 'human spermatozoa' NOT 'animals'. A total of 122 studies were available for analysis.
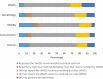
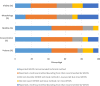
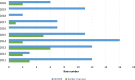
Main results and the role of chance: In total, 70% of the studies cited WHO5 in their methods section. Of the remaining studies, 10% cited the fourth edition of the WHO laboratory manual (WHO4), 7% cited both WHO4 and WHO5, 1% cited the third edition of the WHO laboratory manual (WHO3), and 12% did not cite the WHO at all. Overall methodological agreement with WHO5 recommendations was poor, with the main reason for this lack of agreement being that the research studies did not disclose specific details of the technical methods and equipment used.
Limitations, reasons for caution: In the case of studies that did not disclose any specific technical methods that they used, we did not attempt to contact these authors and so were unable to confirm the agreement between their technical methods and WHO5 recommendations.
Wider implications of the findings: Our findings suggest there is an urgent need to develop strategies to address standardization in reporting the results of a semen analysis for publication. This is particularly timely given the recent publication of WHO6 and ISO standard 23162 for the basic examination of human semen.
Study funding/competing interest(s): There was no funding for this project. C.L.R.B., as an employee of the University of Dundee, serves on the Scientific Advisory board of ExSeed Health (from October 2021, financial compensation to the University of Dundee) and is a scientific consultant for Exscientia (from September 2021, financial compensation to the University of Dundee). C.L.R.B. has previously received a fee from Cooper Surgical for lectures on scientific research methods outside the submitted work (2020) and Ferring for a lecture on male reproductive health (2021). C.L.R.B. is Editor for RBMO.
Trial registration number: N/A.
Keywords: WHO; World Health Organization; andrology; conformance; human spermatozoa; semen analysis; sperm concentration; sperm morphology; sperm motility; standardization.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures
Comment in
-
Reply: How is the compliance of the published studies on WHO5 recommendations for basic semen analysis?Hum Reprod. 2023 Jul 5;38(7):1424-1425. doi: 10.1093/humrep/dead103. Hum Reprod. 2023. PMID: 37253594 No abstract available.
-
How is the compliance of the published studies on WHO5 recommendations for basic semen analysis?Hum Reprod. 2023 Jul 5;38(7):1424. doi: 10.1093/humrep/dead102. Hum Reprod. 2023. PMID: 37253597 No abstract available.
Similar articles
-
A core outcome set for future male infertility research: development of an international consensus.Hum Reprod. 2025 May 1;40(5):865-875. doi: 10.1093/humrep/deaf039. Hum Reprod. 2025. PMID: 40233940 Free PMC article.
-
Myeloperoxidase inhibitor AZD5904 enhances human sperm function in vitro.Hum Reprod. 2021 Feb 18;36(3):560-570. doi: 10.1093/humrep/deaa328. Hum Reprod. 2021. PMID: 33393586
-
Risk of childhood mortality in family members of men with poor semen quality.Hum Reprod. 2017 Jan;32(1):239-247. doi: 10.1093/humrep/dew289. Epub 2016 Dec 6. Hum Reprod. 2017. PMID: 27927843 Free PMC article.
-
Current global status of male reproductive health.Hum Reprod Open. 2024 Apr 12;2024(2):hoae017. doi: 10.1093/hropen/hoae017. eCollection 2024. Hum Reprod Open. 2024. PMID: 38699533 Free PMC article. Review.
-
The sixth edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen: ensuring quality and standardization in basic examination of human ejaculates.Fertil Steril. 2022 Feb;117(2):246-251. doi: 10.1016/j.fertnstert.2021.12.012. Epub 2022 Jan 2. Fertil Steril. 2022. PMID: 34986984 Review.
Cited by
-
Serum FSH levels can predict sperm identification in semen once diagnosed azoospermia.Reprod Fertil. 2025 Mar 3;6(1):e240090. doi: 10.1530/RAF-24-0090. Print 2025 Jan 1. Reprod Fertil. 2025. PMID: 39964111 Free PMC article.
-
Semen Thresholds of Normality Established by the WHO Do Not Reveal Genome Instability-A Potential Occult Male Factor.Genes (Basel). 2023 Jan 17;14(2):239. doi: 10.3390/genes14020239. Genes (Basel). 2023. PMID: 36833166 Free PMC article.
-
Deciphering the role of reactive oxygen species in idiopathic asthenozoospermia.Front Endocrinol (Lausanne). 2025 May 21;16:1505213. doi: 10.3389/fendo.2025.1505213. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40469444 Free PMC article. Review.
-
The Effect of Endurance Exercise on Semen Quality in Male Athletes: A Systematic Review.Sports Med Open. 2024 Jun 11;10(1):72. doi: 10.1186/s40798-024-00739-z. Sports Med Open. 2024. PMID: 38861008 Free PMC article.
-
What is required for better progress in clinical and scientific andrology involving sperm assessments?Asian J Androl. 2024 May 1;26(3):229-232. doi: 10.4103/aja202380. Epub 2024 Jan 16. Asian J Androl. 2024. PMID: 38265240 Free PMC article. No abstract available.
References
-
- Baker M. 1,500 scientists lift the lid on reproducibility. Nature 2016;533:452–454. - PubMed
-
- Björndahl L, Barratt CLR, Fraser LR, Kvist U, Mortimer D.. ESHRE basic semen analysis courses 1995–1999: immediate beneficial effects of standardized training. Hum Rep 2002;17:1299–1305. - PubMed
-
- Björndahl L, Barratt CLR, Mortimer D, Jouannet P.. “How to count sperm properly”: checklist for acceptability of studies based on human semen analysis. Hum Rep 2016;31:227–232. - PubMed
-
- Economist. Trouble at the Lab. 2013. https://www.economist.com/briefing/2013/10/18/trouble-at-the-lab (4 April 2022, date last accessed).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

