Immunogenicity and safety of MenACWY-TT, a quadrivalent meningococcal tetanus toxoid conjugate vaccine recently licensed in the United States for individuals ≥2 years of age
- PMID: 35947774
- PMCID: PMC9746432
- DOI: 10.1080/21645515.2022.2099142
Immunogenicity and safety of MenACWY-TT, a quadrivalent meningococcal tetanus toxoid conjugate vaccine recently licensed in the United States for individuals ≥2 years of age
Erratum in
-
Correction.Hum Vaccin Immunother. 2022 Nov 30;18(6):2133330. doi: 10.1080/21645515.2022.2133330. Epub 2022 Oct 19. Hum Vaccin Immunother. 2022. PMID: 36260932 Free PMC article. No abstract available.
Abstract
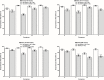
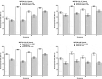
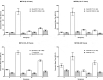
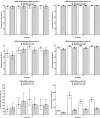
Vaccination offers the best way to prevent invasive meningococcal disease (IMD). As demonstrated in countries with national immunization programs (NIPs) against IMD, meningococcal conjugate vaccines have contributed to significant declines in incidence. Since some meningococcal vaccines are associated with modest immunogenicity in infants, possible immunological interference upon concomitant administration with some pediatric vaccines, and administration errors resulting from improper reconstitution, opportunities for improvement exist. A quadrivalent conjugate vaccine, MenQuadfi® (Meningococcal [Serogroups A, C, Y, and W] Conjugate Vaccine; Sanofi, Swiftwater, Pennsylvania), was approved in 2020 for the prevention of IMD caused by meningococcal serogroups A, C, W, and Y in individuals ≥2 years of age in the United States. Five pivotal studies and one ancillary study supported approval in the United States; clinical trials in infants are ongoing. Data on the immunogenicity and safety of this vaccine are presented, and its potential value in clinical practice is discussed.
Keywords: IMD; Immunogenicity; MenACWY-CRM; MenACWY-D; MenACWY-TT; MenACYW-TT; MenQuadfi; invasive meningococcal disease; meningococcal conjugate vaccine; safety; vaccine recommendations.
Conflict of interest statement
CAR and PO are employees of, and hold stock options in, Sanofi. GSM has been an investigator on clinical trials funded by GlaxoSmithKline, Merck, Novartis, Pfizer, Sanofi, and Seqirus, and has received honoraria from these companies for service on advisory boards. SIP has received honoraria from Sanofi for participation in advisory boards on meningococcal conjugate vaccines, influenza vaccines, and COVID-19 vaccines; grants, consulting fees, and honoraria from Pfizer, Inc. for participation in a Data and Safety Monitoring Board (DSMB) for pneumococcal vaccine studies; and honoraria from Seqirus for participation on advisory committees on influenza and COVID-19 vaccines.
Figures
Similar articles
-
Safety and Immunogenicity of a Quadrivalent Meningococcal Conjugate Vaccine in Healthy Meningococcal-Naïve Children 2-9 Years of Age: A Phase III, Randomized Study.Pediatr Infect Dis J. 2020 Oct;39(10):955-960. doi: 10.1097/INF.0000000000002832. Pediatr Infect Dis J. 2020. PMID: 32852352 Free PMC article. Clinical Trial.
-
A novel vaccine to prevent meningococcal disease beyond the first year of life: an early review of MenACYW-TT.Expert Rev Vaccines. 2021 Sep;20(9):1123-1146. doi: 10.1080/14760584.2021.1964962. Epub 2021 Sep 1. Expert Rev Vaccines. 2021. PMID: 34365870
-
Immunogenicity and safety of a pentavalent meningococcal ABCWY vaccine in adolescents and young adults: an observer-blind, active-controlled, randomised trial.Lancet Infect Dis. 2023 Dec;23(12):1370-1382. doi: 10.1016/S1473-3099(23)00191-3. Epub 2023 Aug 11. Lancet Infect Dis. 2023. PMID: 37579773 Clinical Trial.
-
A new quadrivalent meningococcal tetanus toxoid conjugate vaccine: Menquadfi® (MENACWY-TT).Hum Vaccin Immunother. 2025 Dec;21(1):2516949. doi: 10.1080/21645515.2025.2516949. Epub 2025 Jun 10. Hum Vaccin Immunother. 2025. PMID: 40493501 Free PMC article. Review.
-
Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT): A review of the evidence and expert opinion.Expert Rev Vaccines. 2023 Jan-Dec;22(1):447-456. doi: 10.1080/14760584.2023.2211162. Expert Rev Vaccines. 2023. PMID: 37144288 Review.
References
-
- Mbaeyi S, Duffy J, and McNamara LA. Meningococcal disease. In: Hamborsky J, Kroger A, Wolfe S, editors. Epidemiology and prevention of vaccine-preventable diseases. Washington DC: Public Health Foundation. 207–224; 2015.
-
- Dos Santos Souza I, Ziveri J, Bouzinba-Segard H, Morand P, Bourdoulous S. Meningococcus, this famous unknown. C R Biol. 2021;344:127–143. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
