Subtype-Selective Positive Modulation of KCa2.3 Channels Increases Cilia Length
- PMID: 35947779
- PMCID: PMC9396613
- DOI: 10.1021/acschembio.2c00469
Subtype-Selective Positive Modulation of KCa2.3 Channels Increases Cilia Length
Abstract
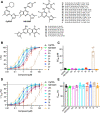
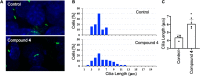
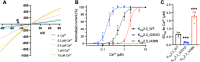
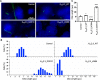
Small-conductance Ca2+-activated potassium (KCa2.x) channels are gated exclusively by intracellular Ca2+. The activation of KCa2.3 channels induces hyperpolarization, which augments Ca2+ signaling in endothelial cells. Cilia are specialized Ca2+ signaling compartments. Here, we identified compound 4 that potentiates human KCa2.3 channels selectively. The subtype selectivity of compound 4 for human KCa2.3 over rat KCa2.2a channels relies on an isoleucine residue in the HA/HB helices. Positive modulation of KCa2.3 channels by compound 4 increased flow-induced Ca2+ signaling and cilia length, while negative modulation by AP14145 reduced flow-induced Ca2+ signaling and cilia length. These findings were corroborated by the increased cilia length due to the expression of Ca2+-hypersensitive KCa2.3_G351D mutant channels and the reduced cilia length resulting from the expression of Ca2+-hyposensitive KCa2.3_I438N channels. Collectively, we were able to associate functions of KCa2.3 channels and cilia, two crucial components in the flow-induced Ca2+ signaling of endothelial cells, with potential implications in vasodilation and ciliopathic hypertension.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Liu Y.; Xie A.; Singh A. K.; Ehsan A.; Choudhary G.; Dudley S.; Sellke F. W.; Feng J. Inactivation of Endothelial Small/Intermediate Conductance of Calcium-Activated Potassium Channels Contributes to Coronary Arteriolar Dysfunction in Diabetic Patients. J. Am. Heart Assoc. 2015, 4, e002062 10.1161/JAHA.115.002062. - DOI - PMC - PubMed
-
- Köhler R.; Degenhardt C.; Kühn M.; Runkel N.; Paul M.; Hoyer J. Expression and function of endothelial Ca(2+)-activated K(+) channels in human mesenteric artery: A single-cell reverse transcriptase-polymerase chain reaction and electrophysiological study in situ. Circ. Res. 2000, 87, 496–503. 10.1161/01.res.87.6.496. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

