Six-Year Results From RELEVANCE: Lenalidomide Plus Rituximab (R2) Versus Rituximab-Chemotherapy Followed by Rituximab Maintenance in Untreated Advanced Follicular Lymphoma
- PMID: 35947804
- PMCID: PMC9553375
- DOI: 10.1200/JCO.22.00843
Six-Year Results From RELEVANCE: Lenalidomide Plus Rituximab (R2) Versus Rituximab-Chemotherapy Followed by Rituximab Maintenance in Untreated Advanced Follicular Lymphoma
Abstract
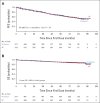
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.The RELEVANCE trial (ClinicalTrials.gov identifier: NCT01650701) showed that lenalidomide plus rituximab (R2) provided similar efficacy to rituximab plus chemotherapy (R-chemo) in patients with advanced-stage, previously untreated follicular lymphoma (FL). We report the second interim analysis of the RELEVANCE trial after 6 years of follow-up. Patients with previously untreated grade 1-3a FL were assigned 1:1 to R2 or R-chemo, followed by rituximab maintenance. Coprimary end points were complete response (confirmed/unconfirmed) at week 120 and progression-free survival (PFS). At median follow-up of 72 months, 6-year PFS was 60% and 59% for R2 and R-chemo, respectively (hazard ratio = 1.03 [95% CI, 0.84 to 1.27]). Six-year overall survival was estimated to be 89% in both groups. Median PFS and overall survival were not reached in either group. Overall response after progression was 61% and 59%, and 5-year estimated survival rate after progression was 69% and 74% in the R2 and R-chemo groups, respectively. The transformation rate per year in the R2 and R-chemo groups was 0.68% and 0.45%, and secondary primary malignancies occurred in 11% and 13% (P = .34), respectively. No new safety signals were observed. R2 continues to demonstrate comparable, durable efficacy and safety versus R-chemo in previously untreated patients with FL and provides an acceptable chemo-free alternative.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures

References
-
- Luminari S, Manni M, Galimberti S, et al. : Response-adapted postinduction strategy in patients with advanced-stage follicular lymphoma: The FOLL12 study. J Clin Oncol 40:729-739, 2022 - PubMed
-
- Rummel MJ, Maschmeyer G, Ganser A, et al. : Bendamustine plus rituximab (B-R) versus CHOP plus rituximab (CHOP-R) as first-line treatment in patients with indolent lymphomas: Nine-year updated results from the StiL NHL1 study. J Clin Oncol 35:7501-7501, 2017
-
- Ghielmini M, Schmitz SF, Cogliatti SB, et al. : Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood 103:4416-4423, 2004 - PubMed
-
- Hainsworth JD, Litchy S, Shaffer DW, et al. : Maximizing therapeutic benefit of rituximab: Maintenance therapy versus re-treatment at progression in patients with indolent non-Hodgkin's lymphoma — A randomized phase II trial of the Minnie pearl cancer research network. J Clin Oncol 23:1088-1095, 2005 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

