Ripretinib Versus Sunitinib in Patients With Advanced Gastrointestinal Stromal Tumor After Treatment With Imatinib (INTRIGUE): A Randomized, Open-Label, Phase III Trial
- PMID: 35947817
- PMCID: PMC9746771
- DOI: 10.1200/JCO.22.00294
Ripretinib Versus Sunitinib in Patients With Advanced Gastrointestinal Stromal Tumor After Treatment With Imatinib (INTRIGUE): A Randomized, Open-Label, Phase III Trial
Abstract
Purpose: Sunitinib, a multitargeted tyrosine kinase inhibitor (TKI), is approved for advanced gastrointestinal stromal tumor (GIST) after imatinib failure. Ripretinib is a switch-control TKI approved for advanced GIST after prior treatment with three or more TKIs, including imatinib. We compared efficacy and safety of ripretinib versus sunitinib in patients with advanced GIST who were previously treated with imatinib (INTRIGUE, ClinicalTrials.gov identifier: NCT03673501).
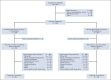
Patients and methods: Random assignment was 1:1 to once-daily ripretinib 150 mg or once-daily sunitinib 50 mg (4 weeks on/2 weeks off) and stratified by KIT/platelet-derived growth factor α mutation and imatinib intolerance. The primary end point was progression-free survival (PFS) by independent radiologic review using modified Response Evaluation Criteria in Solid Tumors version 1.1. Secondary end points included objective response rate by independent radiologic review, safety, and patient-reported outcome measures.
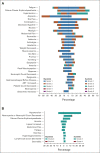
Results: Overall, 453 patients were randomly assigned to ripretinib (intention-to-treat [ITT], n = 226; KIT exon 11 ITT, n = 163) or sunitinib (ITT, n = 227; KIT exon 11 ITT, n = 164). Median PFS for ripretinib and sunitinib (KIT exon 11 ITT) was 8.3 and 7.0 months, respectively (hazard ratio, 0.88; 95% CI, 0.66 to 1.16; P = .36); median PFS (ITT) was 8.0 and 8.3 months, respectively (hazard ratio, 1.05; 95% CI, 0.82 to 1.33; nominal P = .72). Neither was statistically significant. Objective response rate was higher for ripretinib versus sunitinib in the KIT exon 11 ITT population (23.9% v 14.6%, nominal P = .03). Ripretinib was associated with a more favorable safety profile, fewer grade 3/4 treatment-emergent adverse events (41.3% v 65.6%, nominal P < .0001), and better scores on patient-reported outcome measures of tolerability.
Conclusion: Ripretinib was not superior to sunitinib in terms of PFS. However, meaningful clinical activity, fewer grade 3/4 treatment-emergent adverse events, and improved tolerability were observed with ripretinib.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures

Comment in
-
Ripretinib and the Failure to Advance GI Stromal Tumor Therapy in the Age of Precision Medicine.J Clin Oncol. 2022 Dec 1;40(34):3903-3906. doi: 10.1200/JCO.22.01406. Epub 2022 Sep 2. J Clin Oncol. 2022. PMID: 36054848 No abstract available.
References
-
- Rubin BP, Heinrich MC, Corless CL: Gastrointestinal stromal tumour. Lancet 369:1731-1741, 2007 - PubMed
-
- Søreide K, Sandvik OM, Søreide JA, et al. : Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol 40:39-46, 2016 - PubMed
-
- Hirota S, Isozaki K, Moriyama Y, et al. : Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279:577-580, 1998 - PubMed
-
- Heinrich MC, Corless CL, Duensing A, et al. : PDGFRA activating mutations in gastrointestinal stromal tumors. Science 299:708-710, 2003 - PubMed
-
- Szucs Z, Thway K, Fisher C, et al. : Molecular subtypes of gastrointestinal stromal tumors and their prognostic and therapeutic implications. Future Oncol 13:93-107, 2017 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

