Nano-organization of spontaneous GABAergic transmission directs its autonomous function in neuronal signaling
- PMID: 35947950
- PMCID: PMC9392417
- DOI: 10.1016/j.celrep.2022.111172
Nano-organization of spontaneous GABAergic transmission directs its autonomous function in neuronal signaling
Abstract
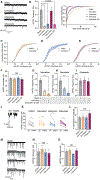
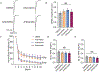
Earlier studies delineated the precise arrangement of proteins that drive neurotransmitter release and postsynaptic signaling at excitatory synapses. However, spatial organization of neurotransmission at inhibitory synapses remains unclear. Here, we took advantage of the molecularly specific interaction of antimalarial artemisinins and the inhibitory synapse scaffold protein, gephyrin, to probe the functional organization of gamma-aminobutyric acid A receptor (GABAAR)-mediated neurotransmission in central synapses. Short-term application of artemisinins severely contracts the size and density of gephyrin and GABAaR γ2 subunit clusters. This size contraction elicits a neuronal activity-independent increase in Bdnf expression due to a specific reduction in GABAergic spontaneous, but not evoked, neurotransmission. The same functional effect could be mimicked by disruption of microtubules that link gephyrin to the neuronal cytoskeleton. These results suggest that the GABAergic postsynaptic apparatus possesses a concentric center-surround organization, where the periphery of gephyrin clusters selectively maintains spontaneous GABAergic neurotransmission facilitating its autonomous function regulating Bdnf expression.
Keywords: CP: Neuroscience; GABAergic transmission; artemisinin; spontaneous neurotransmission; super resolution imaging; synapse nanostructure; synaptic transmission.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

