Investigating the Structure-Activity Relationship of 1,2,4-Triazine G-Protein-Coupled Receptor 84 (GPR84) Antagonists
- PMID: 35948061
- PMCID: PMC9421653
- DOI: 10.1021/acs.jmedchem.2c00804
Investigating the Structure-Activity Relationship of 1,2,4-Triazine G-Protein-Coupled Receptor 84 (GPR84) Antagonists
Abstract
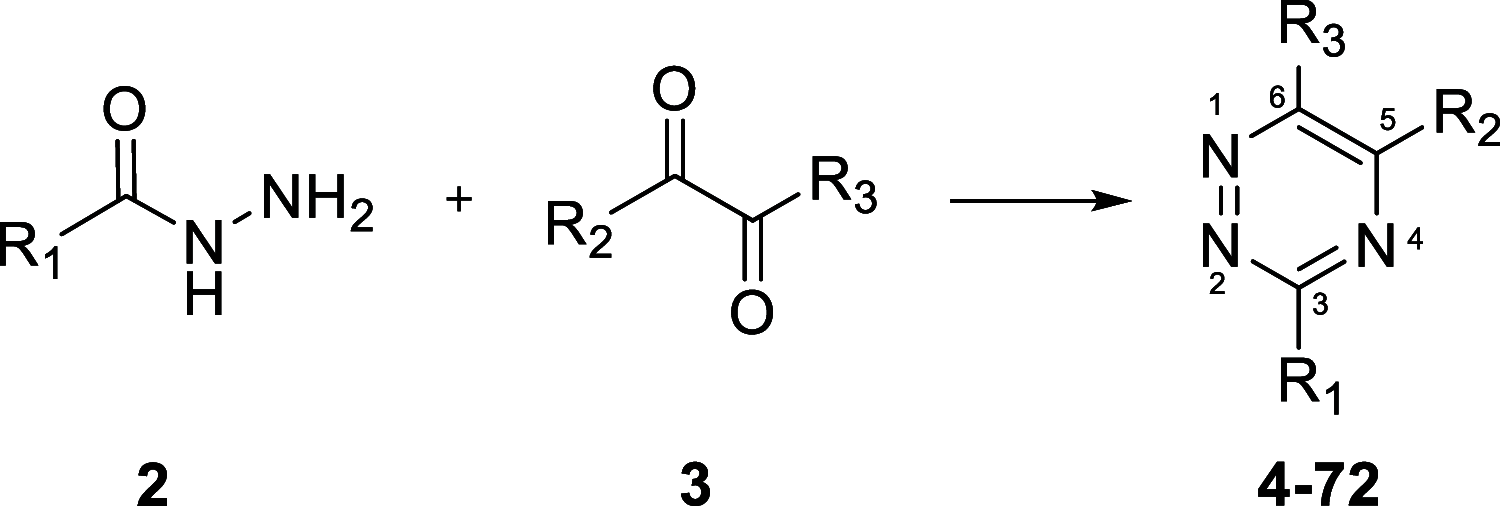
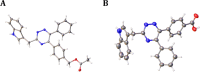
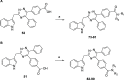
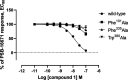
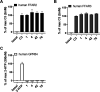
G-protein-coupled receptor 84 (GPR84) is a proinflammatory orphan G-protein-coupled receptor implicated in several inflammatory and fibrotic diseases. Several agonist and antagonist ligands have been developed that target GPR84; however, a noncompetitive receptor blocker that was progressed to phase II clinical trials failed to demonstrate efficacy. New high-quality antagonists are required to investigate the pathophysiological role of GPR84 and to validate GPR84 as a therapeutic target. We previously reported the discovery of a novel triazine GPR84 competitive antagonist 1. Here, we describe an extensive structure-activity relationship (SAR) of antagonist 1 and also present in silico docking with supporting mutagenesis studies that reveals a potential binding pose for this type of orthosteric antagonist. Lead compound 42 is a potent GPR84 antagonist with a favorable pharmacokinetic (PK) profile suitable for further drug development.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Recio C.; Lucy D.; Purvis G. S. D.; Iveson P.; Zeboudj L.; Iqbal A. J.; Lin D.; O’Callaghan C.; Davison L.; Griesbach E.; Russell A. J.; Wynne G. M.; Dib L.; Monaco C.; Greaves D. R. Activation of the immune-metabolic receptor GPR84 enhances inflammation and phagocytosis in macrophages. Front. Immunol. 2018, 9, 141910.3389/fimmu.2018.01419. - DOI - PMC - PubMed
-
- Davenport A. P.; Alexander S. P.; Sharman J. L.; Pawson A. J.; Benson H. E.; Monaghan A. E.; Liew W. C.; Mpamhanga C. P.; Bonner T. I.; Neubig R. R.; Pin J. P.; Spedding M.; Harmar A. J. International union of basic and clinical pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands. Pharmacol. Rev. 2013, 65, 967–986. 10.1124/pr.112.007179. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

