Impaired Mitochondria Promote Aging-Associated Sebaceous Gland Dysfunction and Pathology
- PMID: 35948081
- PMCID: PMC9667715
- DOI: 10.1016/j.ajpath.2022.07.006
Impaired Mitochondria Promote Aging-Associated Sebaceous Gland Dysfunction and Pathology
Abstract
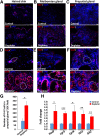
Mitochondrial dysfunction is one of the hallmarks of aging. Changes in sebaceous gland (SG) function and sebum production have been reported during aging. This study shows the direct effects of mitochondrial dysfunction on SG morphology and function. A mitochondrial DNA (mtDNA) depleter mouse was used as a model for introducing mitochondrial dysfunction in the whole animal. The effects on skin SGs and modified SGs of the eyelid, lip, clitoral, and preputial glands were characterized. The mtDNA depleter mice showed gross morphologic and histopathologic changes in SGs associated with increased infiltration by mast cells, neutrophils, and polarized macrophages. Consistently, there was increased expression of proinflammatory cytokines. The inflammatory changes were associated with abnormal sebocyte accumulation of lipid, defective sebum delivery at the skin surface, and the up-regulation of key lipogenesis-regulating genes and androgen receptor. The mtDNA depleter mice expressed aging-associated senescent marker. Increased sebocyte proliferation and aberrant expression of stem cell markers were observed. These studies provide, for the first time, a causal link between mitochondrial dysfunction and abnormal sebocyte function within sebaceous and modified SGs throughout the whole body of the animal. They suggest that mtDNA depleter mouse may serve as a novel tool to develop targeted therapeutics to address SG disorders in aging humans.
Copyright © 2022 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Schneider M.R., Paus R. Sebocytes, multifaceted epithelial cells: lipid production and holocrine secretion. Int J Biochem Cell Biol. 2010;42:181–185. - PubMed
-
- Thody A.J., Shuster S. Control and function of sebaceous glands. Physiol Rev. 1989;69:383–416. - PubMed
-
- Smith K.R., Thiboutot D.M. Thematic review series: skin lipids: sebaceous gland lipids: friend or foe? J Lipid Res. 2008;49:271–281. - PubMed
-
- Drake D.R., Brogden K.A., Dawson D.V., Wertz P.W. Thematic Review Series: skin lipids: antimicrobial lipids at the skin surface. J Lipid Res. 2008;49:4–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

