Orally Ingested Probiotics, Prebiotics, and Synbiotics as Countermeasures for Respiratory Tract Infections in Nonelderly Adults: A Systematic Review and Meta-Analysis
- PMID: 35948276
- PMCID: PMC9776651
- DOI: 10.1093/advances/nmac086
Orally Ingested Probiotics, Prebiotics, and Synbiotics as Countermeasures for Respiratory Tract Infections in Nonelderly Adults: A Systematic Review and Meta-Analysis
Abstract
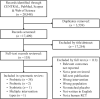
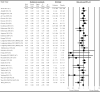
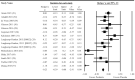
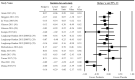
The impact of gut microbiota-targeted interventions on the incidence, duration, and severity of respiratory tract infections (RTIs) in nonelderly adults, and factors moderating any such effects, are unclear. This systematic review and meta-analysis aimed to determine the effects of orally ingested probiotics, prebiotics, and synbiotics compared with placebo on RTI incidence, duration, and severity in nonelderly adults, and to identify potential sources of heterogeneity. Studies were identified by searching CENTRAL, PubMed, Scopus, and Web of Science up to December 2021. English-language, peer-reviewed publications of randomized, placebo-controlled studies that tested an orally ingested probiotic, prebiotic, or synbiotic intervention of any dose for ≥1 wk in adults aged 18-65 y were included. Results were synthesized using intention-to-treat and per-protocol random-effects meta-analysis. Heterogeneity was explored by subgroup meta-analysis and meta-regression. Risk of bias was assessed using the Cochrane risk-of-bias assessment tool for randomized trials version 2 (RoB2). Forty-two manuscripts reporting effects of probiotics (n = 38), prebiotics (n = 2), synbiotics (n = 1) or multiple -biotic types (n = 1) were identified (n = 9179 subjects). Probiotics reduced the risk of experiencing ≥1 RTI (relative risk = 0.91; 95% CI: 0.84, 0.98; P = 0.01), and total days (rate ratio = 0.77; 95% CI: 0.71, 0.83; P < 0.001), duration (Hedges' g = -0.23; 95% CI: -0.39, -0.08; P = 0.004), and severity (Hedges' g = -0.16; 95% CI: -0.29, -0.03; P = 0.02) of RTIs. Effects were relatively consistent across different strain combinations, doses, and durations, although reductions in RTI duration were larger with fermented dairy as the delivery matrix, and beneficial effects of probiotics were not observed in physically active populations. Overall risk of bias was rated as "some concerns" for most studies. In conclusion, orally ingested probiotics, relative to placebo, modestly reduce the incidence, duration, and severity of RTIs in nonelderly adults. Physical activity and delivery matrix may moderate some of these effects. Whether prebiotic and synbiotic interventions confer similar protection remains unclear due to few relevant studies. This trial was registered at https://www.crd.york.ac.uk/prospero/ as CRD42020220213.
Keywords: common cold; coronavirus; dietary supplement; fermentable fiber; gut microbiome; influenza; respiratory illness; respiratory infection.
Published by Oxford University Press on behalf of the American Society for Nutrition 2022.
Figures


References
-
- Cherry DK, Burt CW, Woodwell DA. National Ambulatory Medical Care Survey: 2001 summary. Adv Data. 2003(337):1–44. - PubMed
-
- Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163(4):487–94. - PubMed

