Neurodegeneration in multiple sclerosis
- PMID: 35948371
- PMCID: PMC9839517
- DOI: 10.1002/wsbm.1583
Neurodegeneration in multiple sclerosis
Abstract
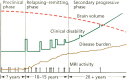
Axonal loss in multiple sclerosis (MS) is a key component of disease progression and permanent neurologic disability. MS is a heterogeneous demyelinating and neurodegenerative disease of the central nervous system (CNS) with varying presentation, disease courses, and prognosis. Immunomodulatory therapies reduce the frequency and severity of inflammatory demyelinating events that are a hallmark of MS, but there is minimal therapy to treat progressive disease and there is no cure. Data from patients with MS, post-mortem histological analysis, and animal models of demyelinating disease have elucidated patterns of MS pathogenesis and underlying mechanisms of neurodegeneration. MRI and molecular biomarkers have been proposed to identify predictors of neurodegeneration and risk factors for disease progression. Early signs of axonal dysfunction have come to light including impaired mitochondrial trafficking, structural axonal changes, and synaptic alterations. With sustained inflammation as well as impaired remyelination, axons succumb to degeneration contributing to CNS atrophy and worsening of disease. These studies highlight the role of chronic demyelination in the CNS in perpetuating axonal loss, and the difficulty in promoting remyelination and repair amidst persistent inflammatory insult. Regenerative and neuroprotective strategies are essential to overcome this barrier, with early intervention being critical to rescue axonal integrity and function. The clinical and basic research studies discussed in this review have set the stage for identifying key propagators of neurodegeneration in MS, leading the way for neuroprotective therapeutic development. This article is categorized under: Immune System Diseases > Molecular and Cellular Physiology Neurological Diseases > Molecular and Cellular Physiology.
Keywords: axonal injury; demyelinating diseases; multiple sclerosis; neurodegeneration; neuroinflammation.
© 2022 The Authors. WIREs Mechanisms of Disease published by Wiley Periodicals LLC.
Figures

References
FURTHER READING
-
- Bernitsas, E. , Bao, F. , Seraji‐Bozorgzad, N. , Chorostecki, J. , Santiago, C. , Tselis, A. , Caon, C. , Zak, I. , Millis, S. , & Khan, O. (2015). Spinal cord atrophy in multiple sclerosis and relationship with disability across clinical phenotypes. Multiple Sclerosis and Related Disorders, 4(1), 47–51. 10.1016/j.msard.2014.11.002 - DOI - PubMed
References
-
- Absinta, M. , Maric, D. , Gharagozloo, M. , Garton, T. , Smith, M. D. , Jin, J. , Fitzgerald, K. C. , Song, A. , Liu, P. , Lin, J.‐P. , Wu, T. , Johnson, K. R. , McGavern, D. B. , Schafer, D. P. , Calabresi, P. A. , & Reich, D. S. (2021). A lymphocyte‐microglia‐astrocyte axis in chronic active multiple sclerosis. Nature, 597(7878), 709–714. 10.1038/s41586-021-03892-7 - DOI - PMC - PubMed
-
- Albrecht, P. , Ringelstein, M. , Müller, A. K. , Keser, N. , Dietlein, T. , Lappas, A. , Foerster, A. , Hartung, H. P. , Aktas, O. , & Methner, A. (2012). Degeneration of retinal layers in multiple sclerosis subtypes quantified by optical coherence tomography. Multiple Sclerosis, 18(10), 1422–1429. 10.1177/1352458512439237 - DOI - PubMed
-
- Arrambide, G. , Rovira, A. , Sastre‐Garriga, J. , Tur, C. , Castilló, J. , Río, J. , Vidal‐Jordana, A. , Galán, I. , Rodríguez‐Acevedo, B. , Midaglia, L. , Nos, C. , Mulero, P. , Arévalo, M. J. , Comabella, M. , Huerga, E. , Auger, C. , Montalban, X. , & Tintore, M. (2018). Spinal cord lesions: A modest contributor to diagnosis in clinically isolated syndromes but a relevant prognostic factor. Multiple Sclerosis, 24(3), 301–312. 10.1177/1352458517697830 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

